Published online Jan 1, 2004. doi: 10.3748/wjg.v10.i1.73
Revised: March 20, 2003
Accepted: April 3, 2003
Published online: January 1, 2004
AIM: Ischemic preconditioning (IP) is a brief ischemic episode, which confers a state of protection against the subsequent long-term ischemia-reperfusion injuries. However, little is known regarding the use of IP before the sustained cold storage and liver transplantation. The present study was designed to evaluate the protective effect of IP on the long-term preservation of liver graft and the prolonged anhepatic-phase injury.
METHODS: Male Sprague-Dawley rats were used as donors and recipients of orthotopic liver transplantation. All livers underwent 10 min of ischemia followed by 10 min of reperfusion before harvest. Rat liver transplantation was performed with the portal vein clamped for 25 min. Tolerance of transplanted liver to the reperfusion injury and liver damage were investigated. The changes in adenosine concentration in hepatic tissue and those of nitric oxide (NO) and tumor necrosis factor (TNF) in serum were also assessed.
RESULTS: Recipients with IP significantly improved their one-week survival rate and liver function, they had increased levels of circulating NO and hepatic adenosine, and a reduced level of serum TNF, as compared to controls. Histological changes indicating hepatic injuries appeared improved in the IP group compared with those in control group. The protective effect of IP was also obtained by administration of adenosine, while blockage of the NO pathway using Nω-nitro-L-arginine methyl ester abolished the protective effect of IP.
CONCLUSION: IP appears to have a protective effect on the long-term preservation of liver graft and the prolonged anhepatic-phase injuries. NO may be involved in this process.
- Citation: Gong JP, Tu B, Wang W, Peng Y, Li SB, Yan LN. Protective effect of nitric oxide induced by ischemic preconditioning on reperfusion injury of rat liver graft. World J Gastroenterol 2004; 10(1): 73-76
- URL: https://www.wjgnet.com/1007-9327/full/v10/i1/73.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i1.73
Liver transplantation is an accepted therapy for patients with end-stage liver diseases[1]. Hepatic ischemia-reperfusion (I/R) injury associated with liver transplantation is an unresolved problem in the clinical practice. Primary non-function of liver graft remains one of the most severe complications of liver transplantation, and poor initial graft function also occurs in one third of cases after liver transplantation. To deal with these complications, no effective treatment can be used but retransplantation[2,3]. Evidently, approaches need to be established to handle or even to avoid these complications.
The underlying mechanisms of cold I/R injuries are still poorly understood. Long-term ischemia of liver graft leads to impairment of liver function and reduction in survival rate of the recipients[4]. On the other hand, anhepatic phase is the most important parameter for orthotopic rat liver transplantation (ORLT), because prolonged anhepatic phase can be associated with an endotoxin-like syndrome caused by the warm intestinal ischemia and is not related to the cold ischemia injuries of the liver[5]. The hepatic reticulo-endothelial system plays a key role in the elimination of endotoxins. The detoxification system is usually deficient in recipients due to liver failure and is completely absent during the anhepatic phase of transplantation. The endotoxin content has been proven to increase markedly in the portal circulation of cirrhotic patients compared to that in the peripheral blood[6]. The hypothermia may affect the ability of Kupffer cells to eliminate endotoxins and to release cytokines, such as tumor-necrosis factor (TNF). It has been shown that TNF is partly responsible for initiation of the lethal toxicity of endotoxins[7].
Ischemic preconditioning (IP) is a process by which a brief ischemic episode confers a state of protection against the subsequent more sustained ischemic insult[8]. Recent studies have demonstrated that a brief ischemia treatment, followed by an episode of reperfusion, can reduce the sustained I/R injury[9,10]. These observations suggest a potential application of IP in liver transplantation. In this study, the protective effect of IP on the long-term preservation of liver grafts and the prolonged anhepatic phase was observed.
Male Sprague-Dawley rats weighing 200 to 230 g supplied by the Center of Experimental Animal in Sichuan University, were used as donors and recipients. Animals were bred in a controlled environment with a 12 hour light/dark cycle. Donor rats were fasted for 12 hours with free access to water before surgery, while the recipients had free access to normal rat chow and water before surgery. This study was approved by Sichuan Bioethics Committee, and the procedures were carried out according to the routine animal-care guidelines.
Liver transplantation was performed according to Kamada’s cuff-technique[11] under anesthesia with ether inhalation. Briefly, the abdomen was opened through a midline incision and the liver was freed from its ligaments with minimal manipulation. The donor bile duct was transected, and a 0.4 cm length of tube was insert into the lumen of the bile duct and secured with a circumferential 5-0 silk suture. The animal was injected intravenously with 50 U of heparin. The liver was perfused through portal vein with an intravenous cannula connected with a syringe with 36 U of heparin in 6 mL of cold saline. The donor liver was placed in the saline bath at 4 °C for 100 min. The cuff preparation of portal vein and infrahepatic vena cava was performed also in the saline-ice bath. After the recipient liver was removed, the donor liver was implanted in the orthotopic position by connecting the suprahepatic vena cava with a running suture, inserting cuffs into the infrahepatic vena cava and the portal vein, and splint tube into the bile duct. The anhepatic phase was estimated to be 25 min for all recipients. ORLT was performed without artery reconstruction.
A total number of 128 rats were randomly divided into 4 groups, 32 for each. 1) Control group, the donor livers were flushed through the portal veins with physiological saline containing heparin only before harvested. 2) IP group, before the donor livers were harvested, the portal vein and hepatic artery were interrupted for 10 min, and the blood flow was restored for 10 min, then the liver was treated as control group. 3) Adenosine group, the donor livers were flushed through the portal vein with physiological saline containing heparin and adenosine (10 mmol/L) only before harvested. 4) Nω-nitro-L-arginine methyl ester (NAME) group, the donor livers were treated as IP group, but NAME (10 mmol/L), an NO synthesis inhibitor, was included in flushing solution. For each group, half of animals were used to investigate the one-week survival rate of recipients, and the remaining animals were for sample collection of blood from infrahepatic vena cava and hepatic tissue after 2 hours of reperfusion.
Serum alanine transaminase (ALT) was measured using an automated analyzer (BECKMAN CX7, Beckman Instruments, Fullerton, CA). Survival of recipients was observed for 7 days after operation.
Values of circulating nitrate and nitrite were determined to reflect serum NO level. Serum was separated by centrifugation and stored at -70 °C before use. Nitrite was measured after enzymatic conversion by nitrate reductase using the Griess reaction, as described by Schmidt[12]. Values obtained represented the sum of serum nitrite and nitrate.
Serum was separated by centrifugation, and concentration of serum TNF was measured by radioimmunoassay. The TNF standard (100 μL, Sigma, ST. Louis, MO. USA) and the serum samples (100 μL) were added separately to appropriate tubes. Two hundred µL of the 0 ng/mL TNF standard was added to each non-specific binding tube. All tubes were added 100 µL of 125I-TNF reagent (Sigma, ST. Louis, MO. USA). TNF antiserum (100 μL) was also added to each tube, except the nonspecific binding tubes. Incubation was done at 4 °C for 24 hours following gentle agitation for 2-3 seconds. With 500 µL of precipitating reagent added, the tubes were vortexed immediately, and incubated for 20 minutes at room temperature (-25 °C). All the tubes were centrifuged at 1500 × g for 25 min, the suspension was dropped out. Radioactivity was read using a gamma counter (262 Factory, Xi’an, Shaanxi, China).
Adenosine standard was purchased from Sigma (St. Louis, MO, USA). High-performance liquid chromatography (HPLC) was performed using a Beckman Gold Nouveau system equipped with a 168 photo-diode-array detector (210 nm; 262 Factory, Xi’an, Shaanxi, China). Satisfactory separation of the marker substances was obtained with a reversed-phase column and eluted at a flow rate of 1 mL/min. Adenosine separation was allowed to precede in a phosphate-buffer solution (331 mmol/L KH2PO4, pH 6.24) containing 3.5% CH3CN and 2.3 mmol/L t-butylamine (TBA). Lyophilized liver tissue was homogenized in 0.5 mL of 0.42 mol/L perchloric acid and incubated for 20 min at 4 °C. The supernatant was separated by centrifugation at 3 000 rpm for 10 min at 0.5 °C, and neutralized with 85 µL/ 200 μL NaOH. After 5 min of centrifugation at 3 000 rpm, 20 µL of the supernatant was subjected to HPLC.
Liver samples were fixed in 10% neutral buffered formalin, embedded in paraffin. Sections of 5 µm in thickness were prepared, stained with hematoxylin and eosin, and observed under a light microscope.
All statistical computations were performed using SPSS software (version 10.0 for Windows 98; SPSS, Inc., Chicago, Illinois). The P values less than 0.05 were considered statistically significant.
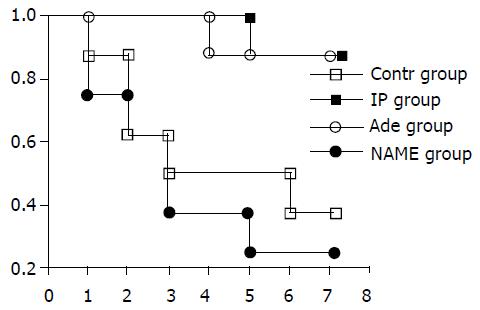
The survival rates of these four groups are shown in Figure 1. Most of the recipients died within 3 days after liver transplantation. The survival rate at day 7 was higher in IP group (87.5%, 7 of 8) and adenosine group (87.5%, 7 of 8) than that in control group (37.5%, 3 of 8) and NAME group (25%, 2 of 8, P < 0.05, Figure1).
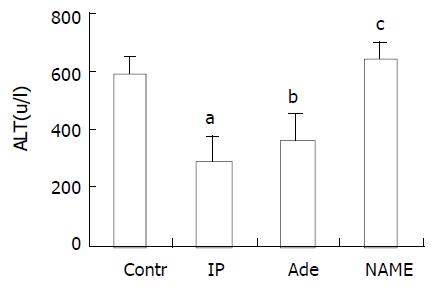
The serum ALT values in control group (588 ± 58 U/L) were significantly higher as compared to those in IP group (287 ± 82 U/L) (P < 0.001). Meanwhile, administration of adenosine also reduced the level of serum ALT (357 ± 93 U/L) as compared to that in control group (P < 0.001). However, with administration of NAME, the response in ALT level to IP was abrogated (634 ± 65 U/L, P > 0.05, Figure 2).
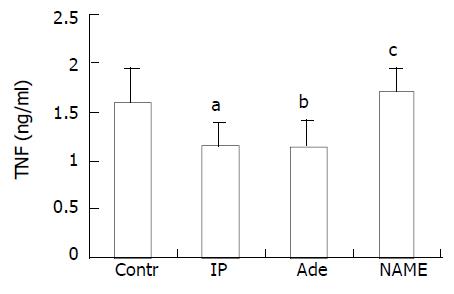
TNF concentration in serum was measured 2 hours after ORLT as described previously[5,6]. In IP group (1.15 ± 0.23 ng/mL) and adenosine group (1.14 ± 0.27 ng/mL), it was significantly lower compared to that in control group (1.59 ± 0.35 ng/mL, P < 0.01). The level in NAME group (1.71 ± 0.23 ng/mL) was as high as that in control group (P > 0.05, Figure 3).
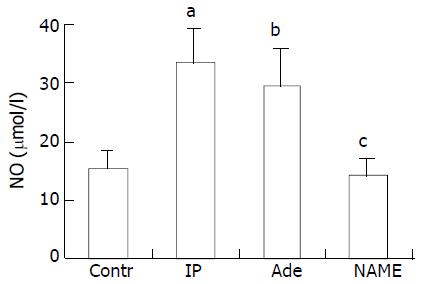
Concentrations of NO were shown to be 32.96 ± 6.10 µmol/L, 29.14 ± 6.49 µmol/L in IP and adenosine groups, respectively, which were significantly higher than that in control group (15.44 ± 2.99 µmol/L, P < 0.001). The value of the recipients in NAME group was 13.74 ± 3.11 μmol/L, which was similar to that in control group (P > 0.05, Figure 4).
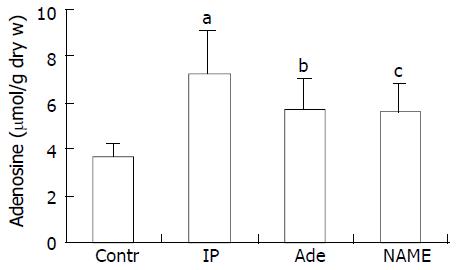
Figure 5 shows the levels of hepatic adenosine at 2 hours after ORLT in liver grafts. Concentrations of tissue adenosine were 7.22 ± 1.83 mol/g, 5.68 ± 1.32 mol/g, and 5.56 ± 1.19 mol/g in liver grafts pretreated with IP, adenosine and IP+NAME, respectively, which were higher than that in the reference liver grafts (3.69 ± 0.54 µmol per gram of dry liver tissue, P < 0.05). Animal survival and liver function were improved after adenosine administration. The protective effect of IP was abrogated in NAME group, though the content of adenosine in the tissue also increased.
Hepatocyte swelling and ballooning were observed in tissues from the control and NAME groups. No identifiable morphological alterations or only minor changes were found in IP and adenosine groups (data not shown).
The ORLT model, established in 1973 by Lee et al.[13], has received wide acceptance in the study of liver transplantation. IP is a procedure originally described during heart transplantation. A brief ischemia treatment followed by reperfusion has been shown to be able to decrease infarct size after subsequent prolonged I/R[8,14,15]. IP has been found to be protective during transplantation of several organs including brain, intestine and skeletal muscle[16-18]. In liver, IP was found to reduce tissue damages and mortality after warm ischemia and cold ischemic storage[5,19].
In the present study, The harvested livers were preserved in saline for 100 min at 4 °Cand the anhepatic phase was selected for 25 minutes, poor outcomes of recipients in control group were observed, and the survival rate 7 days after operation was 37.5% (3/8). In IP group, ALT level was reduced markedly and the survival rate was significantly elevated (87.5%, 7 of 8). The protective effect of IP was further indicated by the morphologic parameters presented.
The effects of a long anhepatic phase on the graft were associated with an endotoxin-like syndrome induced by the prolonged congestion of internal organs. Endotoxemia has been shown to be one of the processes causative for early graft dysfunction[20,21]. This was most probably associated with Kupffer cell activation by splanchnic endotoxin accumulation and release during intestinal congestion/reperfusion[22,23]. During the ORLT with a long anhepatic phase, Kupffer cells were shown to be directly responsible for overproduction of TNF, causing endotoxicosis-like syndrome[24]. The cytotoxic effects of TNF are associated with activation of phospholipaseA2, release of ceramide, formation of reactive oxygen intermediate (ROI) and promotion of cell apoptosis. However, it is not known whether the protective effect of IP in this study was associated with TNF.
The definite mechanism underlying IP is not clear, and there are several explanations about its protective effects. According to some authors, the protective effects were not attributed to blood flow alterations[25,26]. However, the IP protective effect was associated to certain substances produced by ischemic tissue against injury[4]. On the contrary, another study indicated that IP could improve blood flow, and decrease hepatic vascular resistance of liver grafts preserved in cold storage[10], and this was probably related to some potential mediators such as NO and adenosine.
NO was reported to exert a protective effect through inhibiting endothelin synthesis[27], and the upregulating effect of adenosine NO release in endothelial cells was also documented[28]. During ischemia, adenosine is rapidly formed from adenosine triphosphate and reaches high concentrations. Enhanced adenosine level might in turn induce NO synthesis through the activation of adenosine A2 receptors[27]. The functions of the two mediators might lead to an improvement in graft blood flow and accordingly improve liver function. Since the ROIs are known to cause cell injury by promoting the peroxidation of lipids and proteins in cell membranes, it is possible that the antioxidant action of NO might also be involved in the protective effect of IP.
In the present study, the concentrations of NO in serum and adenosine in hepatic tissues of IP group were significantly higher than those in control group. In contrast, the level of serum TNF was greatly reduced after IP treatment. Furthermore, the protective effect of IP was also achieved by administration of adenosine before the donor livers were harvested. Our results showed NAME had negative effects on the protective effect of IP and suppressed NO synthesis, indicating that, in the absence of NO, adenosine was unable to develop protective effects. IP might exert its protective role by inducing the production of NO.
In summary, the data presented suggest that an elevated adenosine level can induce the generation of NO under IP, conferring protection to liver grafts and recipients. IP may be an important approach to reduce the transplantation risk resulted from preservation/reperfusion injuries. Further studies are needed for further understanding of the underlying mechanisms.
Edited by Su Q and Wang XL
| 1. | Lemasters JJ, Thurman RG. Reperfusion injury after liver preservation for transplantation. Annu Rev Pharmacol Toxicol. 1997;37:327-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 237] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 2. | Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 804] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 3. | Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 411] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 4. | Yin DP, Sankary HN, Chong AS, Ma LL, Shen J, Foster P, Williams JW. Protective effect of ischemic preconditioning on liver preservation-reperfusion injury in rats. Transplantation. 1998;66:152-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 151] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 5. | Urata K, Nguyen B, Brault A, Lavoie J, Rocheleau B, Huet PM. Decreased survival in rat liver transplantation with extended cold preservation: role of portal vein clamping time. Hepatology. 1998;28:366-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Lumsden AB, Henderson JM, Kutner MH. Endotoxin levels measured by a chromogenic assay in portal, hepatic and peripheral venous blood in patients with cirrhosis. Hepatology. 1988;8:232-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 322] [Cited by in RCA: 309] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 7. | Fernández ED, Flohé S, Siemers F, Nau M, Ackermann M, Ruwe M, Schade FU. Endotoxin tolerance protects against local hepatic ischemia/reperfusion injury in the rat. J Endotoxin Res. 2000;6:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5406] [Cited by in RCA: 5543] [Article Influence: 142.1] [Reference Citation Analysis (0)] |
| 9. | Arai M, Thurman RG, Lemasters JJ. Contribution of adenosine A(2) receptors and cyclic adenosine monophosphate to protective ischemic preconditioning of sinusoidal endothelial cells against Storage/Reperfusion injury in rat livers. Hepatology. 2000;32:297-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 10. | Ricciardi R, Schaffer BK, Kim RD, Shah SA, Donohue SE, Wheeler SM, Quarfordt SH, Callery MP, Meyers WC, Chari RS. Protective effects of ischemic preconditioning on the cold-preserved liver are tyrosine kinase dependent. Transplantation. 2001;72:406-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Kamada N, Calne RY. A surgical experience with five hundred thirty liver transplants in the rat. Surgery. 1983;93:64-69. [PubMed] |
| 12. | Moshage H, Kok B, Huizenga JR, Jansen PL. Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem. 1995;41:892-896. [PubMed] |
| 13. | Lee S, Charters AC, Chandler JG, Orloff MJ. A technique for orthotopic liver transplantation in the rat. Transplantation. 1973;16:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 102] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Martin HB, Walter CL. Preconditioning: an endogenous defense against the insult of myocardial ischemia. Anesth Analg. 1996;83:639-645. [PubMed] |
| 15. | Schwarz ER, Whyte WS, Kloner RA. Ischemic preconditioning. Curr Opin Cardiol. 1997;12:475-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Heurteaux C, Lauritzen I, Widmann C, Lazdunski M. Essential role of adenosine, adenosine A1 receptors, and ATP-sensitive K+ channels in cerebral ischemic preconditioning. Proc Natl Acad Sci USA. 1995;92:4666-4670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 428] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 17. | Schroeder CA, Lee HT, Shah PM, Babu SC, Thompson CI, Belloni FL. Preconditioning with ischemia or adenosine protects skeletal muscle from ischemic tissue reperfusion injury. J Surg Res. 1996;63:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 68] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Hotter G, Closa D, Prados M, Fernández-Cruz L, Prats N, Gelpí E, Roselló-Catafau J. Intestinal preconditioning is mediated by a transient increase in nitric oxide. Biochem Biophys Res Commun. 1996;222:27-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 143] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 19. | Arai M, Thurman RG, Lemasters JJ. Ischemic preconditioning of rat livers against cold storage-reperfusion injury: role of nonparenchymal cells and the phenomenon of heterologous preconditioning. Liver Transpl. 2001;7:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Clavien PA, Harvey PR, Strasberg SM. Preservation and reperfusion injuries in liver allografts. An overview and synthesis of current studies. Transplantation. 1992;53:957-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 614] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 21. | Zipfel A, Schenk M, You MS, Lauchart W, Bode C, Viebahn R. Endotoxemia in organ donors: graft function following liver transplantation. Transpl Int. 2000;13 Suppl 1:S286-S287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Maring JK, Klompmaker IJ, Zwaveling JH, van Der Meer J, Limburg PC, Slooff MJ. Endotoxins and cytokines during liver transplantation: changes in plasma levels and effects on clinical outcome. Liver Transpl. 2000;6:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Miyata T, Yokoyama I, Todo S, Tzakis A, Selby R, Starzl TE. Endotoxaemia, pulmonary complications, and thrombocytopenia in liver transplantation. Lancet. 1989;2:189-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Urata K, Brault A, Rocheleau B, Huet PM. Role of Kupffer cells in the survival after rat liver transplantation with long portal vein clamping times. Transpl Int. 2000;13:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Cohen MV, Liu GS, Downey JM. Preconditioning causes improved wall motion as well as smaller infarcts after transient coronary occlusion in rabbits. Circulation. 1991;84:341-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 180] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Peralta C, Hotter G, Closa D, Gelpí E, Bulbena O, Roselló-Catafau J. Protective effect of preconditioning on the injury associated to hepatic ischemia-reperfusion in the rat: role of nitric oxide and adenosine. Hepatology. 1997;25:934-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 262] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 27. | Peralta C, Hotter G, Closa D, Prats N, Xaus C, Gelpí E, Roselló-Catafau J. The protective role of adenosine in inducing nitric oxide synthesis in rat liver ischemia preconditioning is mediated by activation of adenosine A2 receptors. Hepatology. 1999;29:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 161] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 28. | Smits P, Williams SB, Lipson DE, Banitt P, Rongen GA, Creager MA. Endothelial release of nitric oxide contributes to the vasodilator effect of adenosine in humans. Circulation. 1995;92:2135-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 154] [Article Influence: 5.1] [Reference Citation Analysis (0)] |