Published online Jan 1, 2004. doi: 10.3748/wjg.v10.i1.26
Revised: March 1, 2003
Accepted: March 18, 2003
Published online: January 1, 2004
AIM: To construct and identify the recombinant vectors carrying herpes simplex virus thymidine kinase (HSV-TK) and tumor necrosis factor alpha (TNF-α) or interleukin-2 (IL-2) genes expressed in gastric carcinoma cell line SGC7901.
METHODS: The fragments of HSV-TK, internal ribosome entry sites (IRES) and TNF-α or IL-2 genes were inserted in a TK-IRES-TNF-α or TK-IRES-IL-2 order into pEGFP-N3 and pLXSN to generate the therapeutic vectors pEGFP-TT, pEGFP-TI, pL(TT)SN and pL(TI)SN respectively, which were structurally confirmed by the digestion analysis of restriction endonuclease. The former two plasmids were used for the transient expression of recombinant proteins in the target cells while pL(TT)SN and pL(TI)SN were transfected into SGC7901 cells by lipofectamine for the stable expression of objective genes through G418 selection. The protein products expressed transiently and stably in SGC7901 cells by the constructed vectors were confirmed by fluorescent microscopy and Western blot respectively.
RESULTS: The inserted fragments in all constructed plasmids were structurally confirmed to be consistent with that of the published data. In the transient expression, both pEGFP-TT and pEGFP-TI were shown expressed in nearly 50% of the transfected SGC7901 cells. Similarly, the G418 selected vectors PL(TT)SN and PL(TI)SN were confirmed to be successful in the stable expression of the objective proteins in the target cells.
CONCLUSION: The constructed recombinant vectors in the present study that can express the suicide gene TK in combination with cytokines genes may serve as the potential tools to perform more effective investigations in future for the gene therapy of gastric carcinoma.
- Citation: Zhang JH, Wan MX, Yuan JY, Pan BR. Construction and identification of recombinant vectors carrying herpes simplex virus thymidine kinase and cytokine genes expressed in gastric carcinoma cell line SGC7901. World J Gastroenterol 2004; 10(1): 26-30
- URL: https://www.wjgnet.com/1007-9327/full/v10/i1/26.htm
- DOI: https://dx.doi.org/10.3748/wjg.v10.i1.26
Gastric cancer is one of the most common malignancies both in China and abroad[1-7]. Despite the improvements in surgical techniques, radiation and chemotherapeutic regimens, the disease remains a great challenge. Most patients still die finally of their disease, even after apparent “curative resection”. In recent years, the promising conception of gene therapy has been advocated in a hope to deal with the malignant diseases more effectively. One of the landmark discoveries for this therapeutic strategy is the transfer of suicide genes, such as HSV-TK, into the tumor cells, which has been shown to exert antitumor efficacy on a variety of cancer cells[8-11]. The expressed HSV-TK/ganciclovir (GCV) system can not only inhibit the DNA synthesis of the target cells but also produce a bystander effect against tumors[12-16]. However, these effects have been found to be unstable in some of the transfected cells, which may result in a decreased efficiency in the treatment of malignancies. The use of tissue-specific vectors to deliver genes and combination of TK with cytokine genes may improve the efficacy of the antitumor effects[17-20]. In the present study, therefore, we tried to construct and identify the recombinant vectors containing HSV-TK, IRES in combination with cytokine genes, TNF-α or IL-2, in an attempt to establish more effective recombinants for the gene therapy of gastric malignancies.
Reagents Restriction enzymes (EcoR I, Hpa I, Xho I and BamHI), T4 DNA ligase and Taq DNA polymerase were purchased from Gibco Co., USA. Lipofectamine was supplied by Boeringer Mannheim Co., Mannheim, Germany. Monoclonal antibodies of mouse anti-IL-2 and anti-TNF-α proteins and horseradish peroxidate-conjugated antimouse immunoglobulin were purchased from Zhongshan Co., Shanghai, China.
PCR primers The primer sequences used in the study were designed according to that of the individual genes (Table 1) with the modification of adding the recognition site sequences for the corresponding restriction enzymes (EcoR I, Hpa I, Xho I and BamH I), and synthesized by Shanghai GeneCore Bio Technologies Co.
| Genes | Primer sequences | Fragment length |
| TK | Forward: 5’-GC GAA TTC ATG GCT TCG TAC CCC TGC CAT C-3’ | 1 128 bp |
| Reverse: 5’-GC GTT AAC TTA AGC CTC CCC CAT CTC CCG G-3 | ||
| IL-2 | Forward: 5’-GC CTC GAG ATG TAC AGG ATG CAA CTC CTG-3’ | 456 bp |
| Reverse: 5’-GC GGA TTC TTA AGT CAG TGT TGA GAT GAT GC-3’ | ||
| TNF-α | Forward: 5’-GC CTC GAG ATG GTC AGA TCA TCT TCT CGA AC -3’ | 474 bp |
| Reverse: 5’-GC GGA TCC TTA CAG GGC AAT GAT CCC AAA -G-3’ | ||
| IRES | Forward: 5’-GC GTT AAC AAT TCC GCC CCT CTC CCT CCC CC-3’ | 585 bp |
| Reverse: 5’-GC CTC GAG AAT AGT AGC ACA AAA AGT TTC C-3’ |
Plasmids and cell lines Plasmid pBluescript-TK was provided by Shanghai Institute of Biochemistry. pIRZA1neo with 585 bp IRES sequence[21,22] was purchased from Invitrogen Co., USA. The plasmid PT7-TNF-α, pEGFP-N3 carrying green fluorescent protein (GFP) gene, pGEM-T-Easy vector and E.coli JM109 were provided by Orthopedic Oncology Institute of PLA, Xi’an, China. The retroviral expressing vector pLXSN was provided by Dr. Yu Bing from Fourth Military Medical University, Xi’an, China. IL-2 cDNA was made from human peripheral blood by RT-PCR.
The gastric carcinoma cell line SGC7901 was provided by Shanghai Institute of Biochemistry. Virus packaging cell PA317 and NIH3T3 cell lines were provided by Dr. Yu Bing. Cells were maintained in RPMI 1640 medium supplemented with 10% FBS (Hangzhou, Sijiqing Biotech Company), 2 mM L-glutamine, 100 units /mL penicillin and 100 μg/mL streptomycin. The PA317 was used as the packaging cell and the NIH 3T3 cells were used to assay the virus titre. The cell cultures were maintained at 37 °C in a humidified atmosphere with 5% CO2.
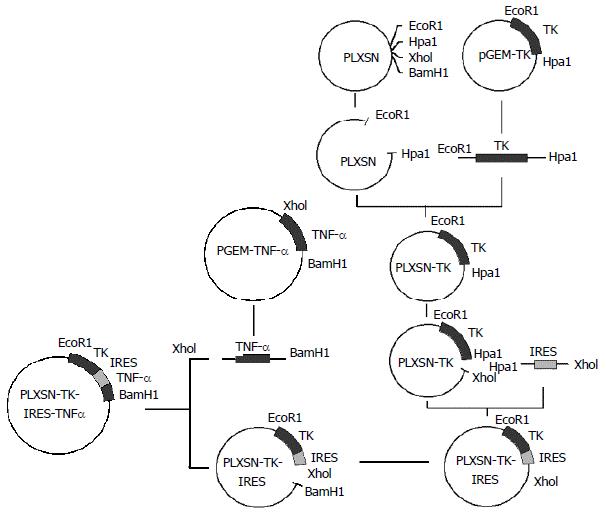
Construction of the recombinant vectors The recombinant vectors were constructed with routine molecular cloning techniques[23-25]. The DNA fragments of HSV-TK, IRES, TNF-α and IL-2 were obtained by PCR amplification with specific primers from their corresponding templates. For the transient expression of the recombinant genes, the fragments of HSV-TK, IRES, TNF-α and the fragments of HSV-TK, IRES, IL-2 were cloned into pEGFP-N3 to generate pEGFP-TT and pEGFP-TI respectively. Similarly, those fragments were separately inserted into pLXSN to generate plasmid PL (TT) SN and PL (TI) SN as shown in Figure 1 for the selection of the stable expression vectors. The structure of all these constructed vectors was confirmed by the digestion analysis of restriction endonuclease.
Transient expression The transient expression of recombinants was performed according to the literature[26]. The constructed vectors pEGFP-TT and pEGFP-TI, and the control plasmid were transfected into SGC7901 cells with a routine protocol by lipofectamine. Twenty hours after the transfection, cells were harvested and the expressed marker protein GFP fused with the objective genes were detected under a fluorescent microscope.
Stable expression The plasmids pL(TT)SN, pL(TI)SN and pL(TK)SN were transfected respectively into PA317 cells with lipofectamine (Gibco) according to the manufacturer’s instruction. After 48 h of transfection, G418 (Promega) was added to the culture media at a concentration of 500 mg·L-1 to select G418-resistant colonies. After 2-weeks’ culture with the changing of the G418-containing media every 3 days, the supernatant of G418-resistant colony was collected and diluted to different concentrations to infect NIH3T3 cells, which was further undergone the G418 selection for 2w when the G418-resistant NIH3T3 colonies were counted for the determination of viral titre. The viral titer of pL(TT)SN, pL(TI)SN, pL(TK) SN and empty plasmid pLXSN were 5 × 108 CFU/L, 6 × 108 CFU/L, 1 × 109 CFU/L and 1 × 109 CFU/L respectively.
For the stable expression of recombinants, a total number of 5 × 105 SGC7901 cells were incubated in a 6-well plate for 24 h, then rinsed with serum-free RPMI 1640 medium twice and incubated with 100 μl supermatant of G418-resistant PA317 colony for 3 h. After 4-weeks’ cultivation, the G418-resistant colonies designated as SGC/TK-TNF-α, SGC/TK-IL-2, SGC/TK and SGC/0 respectively were used to confirm the objective gene expression by Western blot analysis.
Western blotting analysis The SGC/TT and SGC/TI cells were incubated respectively in the six-well plates at a density of 2.5 × 105 cells/well for 24 h, followed by a further cultivation of 48 h with the culture medium replaced with 1 mL of serum-free RPMI 1640. Then the serum-free medium was totally collected, concentrated in a microconcentrator to 20 μl and subjected to electrophoresis on a 120 g·L-1 SDS/PAGE gel. Proteins were transferred to a nitrocellulose membrane and incubated overnight in 50 mL·L-1 fat free milk in PBS at 4 °C. After washed in 10 mL·L-1 fat free milk, the membrane was incubated with monoclonal antibody of mouse anti-rhIL-2 or anti-TNF-α, followed by incubation with horseradish peroxidate-conjugated antimouse immunoglobulin. Proteins were detected by using the ECL kit according to the manufacturer’s protocol (Amersham).
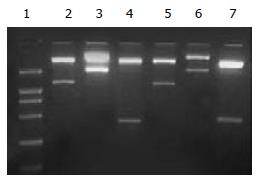
The segment analysis by restriction endonuclease digestion confirmed that the inserted gene sequences in all of the constructed plasmids were structurally consistent with that of the published data. The inserted fragments in pEGFP-TI and pEGFP-TT were identified as shown in Figure 2.
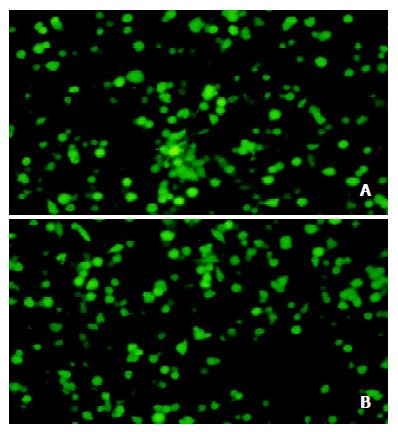
Twenty hours after the transfection, the GFP fluorescence was detected under fluorescent microscope in nearly 50% of the total cells transfected with pEGFP-TT or pEGFP-TI. The fluorescence was gradually increased with time and peaked at 72 h, which indicated that the TK-IRES-IL-2 and TK-IRES-TNF-α were transiently expressed in SGC7901 cells (Figure 3 A and B).
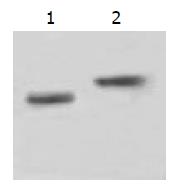
The stable expression of recombinant proteins in SGC7901 cells was confirmed by Western blotting, in which two distinct bands of 15ku and 17ku were observed on the nitrocellulose membrane, corresponding to the fragment sizes of IL-2 and TNF-α respectively (Figure 4).
Gene therapy has become a promising strategy for the treatment of gastric cancer[27-31], in which the transfer of suicide genes into tumor cells has emerged as an attractive modality for the selective elimination of cancer cells[14-16,30,31]. The suicide genes encode non-mammalian enzymes that can convert nontoxic prodrugs into cellular toxic metabolites. The most widely used suicide gene is the HSV-TK/ganciclovir (GCV) system that can convert prodrug GCV into GCV monophosphate. The latter is further phosphorated by cellular kinase to form GCV triphosphate, a toxic substance that can inhibit cellular DNA synthesis and lead to cell death. Besides, the “bystander effect” induced by TK gene can also enhance the tumor-killing capacity of the HSV-TK/GCV system[16-20,32]. Because of the antitumor properties exerted by certain cytokines such as IL-2 and TNF-α, it is believed that the gene therapy combining cytokine with TK suicide gene would be more beneficial and effective for the treatment of cancers, which has been strongly supported by some of the recently published literatures[33]. However, there are fewer reports of this therapeutic strategy applied to the antitumor study of gastric malignancy.
Construction of a bicistronic retroviral vector with an internal ribosome entry site (IRES)[34,35] allows the simultaneous expression of two genes from a single transcript, which has been demonstrated to be efficient. However, the expressive levels of the objective protein are found lower in some circumstance. Five different genes, including human IL-2, IL-4, granulocyte macrophage stimulating factor, HSV-TK and hepatitis C virus core gene, have been tested using the modified vector for the gene transfer[36,37]. The new bicistronic vector, modified by abolishing the functional viral gag initiation codon and keeping it before the 5’ end to the first initiation codon of the transduced gene, has made the protein expression greatly increased compared with the original vector. As the RNA levels and splicing patterns of these two vectors remain similar, the improvement was most likely at the translation level. Thus, the incorporation of the internal ribosome entry site sequence into a proper location of the retroviral vector for gene therapy represents a promising strategy to facilitate the simultaneous and efficient expression of several genes from the same promoter[21,22].
In the present study, we employed the strategy to construct our expression vectors, in which the IRES gene was cloned in TK-IRES-TNF-α or TK-IRES-IL-2 order and the recombinants were constructed in combination with TK and cytokine genes. In the transient expression, the constructed vectors pEGFP-TT and pEGFP-TI were shown to be effectively expressed in vitro as demonstrated by the appearance of the fusing fluorescent protein GFP[26,38] in nearly 50% of the transfected SGC7901 cells. Similarly, in the experiment of stable expression, the G418 selected vectors PL(TT)SN and PL(TI)SN were also confirmed to be successful in both of the transfection into the target cells and the expression of the objective proteins. All of these results indicate that the expressive vectors constructed in the present study may serve as the potential tools to perform more effective investigations for the gene therapy of gastric carcinoma in future. Further studies are therefore needed to elucidate and characterize the antitumor effects of these constructed vectors on the transfected SGC7901 cells.
Edited by Zhu LH
| 1. | Xu CT, Huang LT, Pan BR. Current gene therapy for stomach carcinoma. World J Gastroenterol. 2001;7:752-759. [PubMed] |
| 2. | Song ZJ, Gong P, Wu YE. Relationship between the expression of iNOS,VEGF,tumor angiogenesis and gastric cancer. World J Gastroenterol. 2002;8:591-595. [PubMed] |
| 3. | Tao HQ, Zou SC. Effect of preoperative regional artery chemotherapy on proliferation and apoptosis of gastric carcinoma cells. World J Gastroenterol. 2002;8:451-454. [PubMed] |
| 4. | Han Y, Han ZY, Zhou XM, Shi R, Zheng Y, Shi YQ, Miao JY, Pan BR, Fan DM. Expression and function of classical protein kinase C isoenzymes in gastric cancer cell line and its drug-resistant sublines. World J Gastroenterol. 2002;8:441-445. [PubMed] |
| 5. | Wang X, Lan M, Shi YQ, Lu J, Zhong YX, Wu HP, Zai HH, Ding J, Wu KC, Pan BR. Differential display of vincristine-resistance-related genes in gastric cancer SGC7901 cell. World J Gastroenterol. 2002;8:54-59. [PubMed] |
| 6. | Shen LZ, Wu WX, Xu DH, Zheng ZC, Liu XY, Ding Q, Hua YB, Yao K. Specific CEA-producing colorectal carcinoma cell killing with recombinant adenoviral vector containing cytosine deaminase gene. World J Gastroenterol. 2002;8:270-275. [PubMed] |
| 7. | Thomas AL, Steward WP. Recent advances in the nonsurgical treatment of upper gastrointestinal tract tumors. Expert Rev Anticancer Ther. 2001;1:258-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Narita M, Bahar R, Hatano M, Kang MM, Tokuhisa T, Goto S, Saisho H, Sakiyama S, Tagawa M. Tissue-specific expression of a suicide gene for selective killing of neuroblastoma cells using a promoter region of the NCX gene. Cancer Gene Ther. 2001;8:997-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | van Dillen IJ, Mulder NH, Vaalburg W, de Vries EF, Hospers GA. Influence of the bystander effect on HSV-tk/GCV gene therapy. A review. Curr Gene Ther. 2002;2:307-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 97] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 10. | Nowak AK, Lake RA, Kindler HL, Robinson BW. New approaches for mesothelioma: biologics, vaccines, gene therapy, and other novel agents. Semin Oncol. 2002;29:82-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 82] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Qiao J, Doubrovin M, Sauter BV, Huang Y, Guo ZS, Balatoni J, Akhurst T, Blasberg RG, Tjuvajev JG, Chen SH. Tumor-specific transcriptional targeting of suicide gene therapy. Gene Ther. 2002;9:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 87] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Engelmann C, Heslan JM, Fabre M, Lagarde JP, Klatzmann D, Panis Y. Importance, mechanisms and limitations of the distant bystander effect in cancer gene therapy of experimental liver tumors. Cancer Lett. 2002;179:59-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Adachi Y, Matsubara S, Muramatsu T, Curiel DT, Reynolds PN. Midkine promoter-based adenoviral suicide gene therapy to midkine-positive pediatric tumor. J Pediatr Surg. 2002;37:588-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Pulkkanen KJ, Laukkanen JM, Fuxe J, Kettunen MI, Rehn M, Kannasto JM, Parkkinen JJ, Kauppinen RA, Pettersson RF, Yla-Herttuala S. The combination of HSV-tk and endostatin gene therapy eradicates orthotopic human renal cell carcinomas in nude mice. Cancer Gene Ther. 2002;9:908-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Floeth FW, Shand N, Bojar H, Prisack HB, Felsberg J, Neuen-Jacob E, Aulich A, Burger KJ, Bock WJ, Weber F. Local inflammation and devascularization--in vivo mechanisms of the "bystander effect" in VPC-mediated HSV-Tk/GCV gene therapy for human malignant glioma. Cancer Gene Ther. 2001;8:843-851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Warren P, Song W, Holle E, Holmes L, Wei Y, Li J, Wagner T, Yu X. Combined HSV-TK/GCV and secondary lymphoid tissue chemokine gene therapy inhibits tumor growth and elicits potent antitumor CTL response in tumor-bearing mice. Anticancer Res. 2002;22:599-604. [PubMed] |
| 17. | Guan J, Ma L, Wei L. Characteristics of ovarian cancer cells transduced by the bicistronic retroviral vector containing GM-CSF and HSV-TK genes. Chin Med J (Engl). 2001;114:147-151. [PubMed] |
| 18. | Hall SJ, Canfield SE, Yan Y, Hassen W, Selleck WA, Chen SH. A novel bystander effect involving tumor cell-derived Fas and FasL interactions following Ad.HSV-tk and Ad.mIL-12 gene therapies in experimental prostate cancer. Gene Ther. 2002;9:511-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Boucher PD, Ostruszka LJ, Murphy PJ, Shewach DS. Hydroxyurea significantly enhances tumor growth delay in vivo with herpes simplex virus thymidine kinase/ganciclovir gene therapy. Gene Ther. 2002;9:1023-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Hayashi K, Hayashi T, Sun HD, Takeda Y. Contribution of a combination of ponicidin and acyclovir/ganciclovir to the antitumor efficacy of the herpes simplex virus thymidine kinase gene therapy system. Hum Gene Ther. 2002;13:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Ulrich-Vinther M, Carmody EE, Goater JJ, S balle K, O'Keefe RJ, Schwarz EM. Recombinant adeno-associated virus-mediated osteoprotegerin gene therapy inhibits wear debris-induced osteolysis. J Bone Joint Surg Am. 2002;84-A:1405-1412. [PubMed] |
| 22. | Wong ET, Ngoi SM, Lee CG. Improved co-expression of multiple genes in vectors containing internal ribosome entry sites (IRESes) from human genes. Gene Ther. 2002;9:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Cao MM, Pan W, Chen QL, Ma ZC, Ni ZJ, Wu XL, Wu WB, Pan X, Cao GW, Qi ZT. Construction of the eukaryotic expression vector expressing the fusion protein of human endostatin pro-tein and IL3 signal peptide. Shijie Huaren Xiaohua Zazhi. 2001;9:43-46. |
| 24. | Pan X, Ke CW, Pan W, Wu WB, Zhang B, He X, Cao GW, Qi ZT. Construction of eukaryotic expression vector carrying IFN-β gene under control of human HBV promoter. Shijie Huaren Xiaohua Zazhi. 2000;8:520-523. |
| 25. | Zhang J, Liu YF, Yang SJ, Sun ZW, Qiao Q, Zhang SZ. Construc-tion and expression of mouse/humanized scFv and their fusion to humanized mutant TNFα against hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi. 2000;8:616-620. |
| 26. | Cheng H, Liu YF, Zhang HZ, Shen WA, Zhang SZ. Construction and expression of anti-HCC immunotoxin of sFv-TNF-α and GFP fusion proteins. Shijie Huaren Xiaohua Zazhi. 2001;9:640-644. |
| 27. | Chen JP, Lin C, Xu CP, Zhang XY, Wu M. The therapeutic effects of recombinant adenovirus RA538 on human gastric carcinoma cells in vitro and in vivo. World J Gastroenterol. 2000;6:855-860. [PubMed] |
| 28. | Feng RH, Zhu ZG, Li JF, Liu BY, Yan M, Yin HR, Lin YZ. Inhibition of human telomerase in MKN-45 cell line by antisense hTR expression vector induces cell apoptosis and growth arrest. World J Gastroenterol. 2002;8:436-440. [PubMed] |
| 29. | Yu ZC, Ding J, Pan BR, Fan DM, Zhang XY. Expression and bioactivity identification of soluble MG7 scFv. World J Gastroenterol. 2002;8:99-102. [PubMed] |
| 30. | Guo SY, Gu QL, Liu BY, Zhu ZG, Yin HR, Lin YZ. Experimental study on the treatment of gastric cancer by TK gene combined with mIL-2 gene. Shijie Huaren Xiaohua Zazhi. 2000;8:974-978. |
| 31. | Huang H, Wang A. [The adenovirus-mediated HSV-TK/GCV suicide gene system in the treatment of tongue carcinoma cell line]. Zhonghua Kouqiang Yixue Zazhi. 2001;36:457-460. [PubMed] |
| 32. | Gao G, Huang T, Chen S. [In vitro and in vivo bystander effect of adenovirus-mediated transfer of the herpes simplex virus thymidine kinase gene]. Zhonghua Waike Zazhi. 2002;40:301-303. [PubMed] |
| 33. | Majumdar AS, Zolotorev A, Samuel S, Tran K, Vertin B, Hall-Meier M, Antoni BA, Adeline E, Philip M, Philip R. Efficacy of herpes simplex virus thymidine kinase in combination with cytokine gene therapy in an experimental metastatic breast cancer model. Cancer Gene Ther. 2000;7:1086-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 34. | Kobayashi T, Kida Y, Kaneko T, Pastan I, Kobayashi K. Efficient ablation by immunotoxin-mediated cell targeting of the cell types that express human interleukin-2 receptor depending on the internal ribosome entry site. J Gene Med. 2001;3:505-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 35. | Royal RE, Kershaw MH, Reeves ME, Wang G, Daly T, Treisman J, Lam J, Hwu P. Increased functional expression of transgene in primary human lymphocytes using retroviral vectors modified with IRES and splicing motifs. Gene Ther. 2002;9:1085-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 36. | Wang SI, Mukhtar H. A high-efficiency translational control element with potential for cancer gene therapy. Int J Oncol. 2002;20:1269-1274. [PubMed] |
| 37. | Qiao J, Roy V, Girard MH, Caruso M. High translation efficiency is mediated by the encephalomyocarditis virus internal ribosomal entry sites if the natural sequence surrounding the eleventh AUG is retained. Hum Gene Ther. 2002;13:881-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 38. | Zhang X, Wu J, Li X, Fu L, Gao D, Bai H, Liu X. [Effects of recombinant human bone morphogenic protein-2 and hyaluronic acid on invasion of brain glioma in vivo]. Zhonghua Yixue Zazhi. 2002;82:90-93. [PubMed] |