INTRODUCTION
Human and animals normally maintain homeostasis of ion concentration, pH, body fluid and osmotic pressure. Homeostasis would markedly change, if human and animals drink excessive hypernatric fluids. Variations in osmotic pressure of extracellular fluid induce changes in cell volume that result in profound alteration of cell function and signal transduction between cells by modifying both extracellular and interacellular spatial arrangement and solute concentrations. Osmotic stimuli are resulted from the integration of multiple sensory inputs including peripheral and central osmoreceptors[1,2]. Peripheral osmoreceptors are mainly localized in mesenteric vasculature of the upper small intestine and hepatic portal vein[3]. The osmotic, immune, and noxious information from these peripheral receptors would transmit to medullary nuclei (the medullary visceral zone-MVZ that includes nucleus of the tractus solitarius and ventrolateral medulla) via vagus[4-8]. The effects of infusions of salt and water on the stomach can be mostly prevented by damage of the splanchnic or hepatic vagal nerves[3]. The neurons in medullary nucleus relay information and reach the supraoptic nucleus (SON) and paraventricullar nucleus (PVN) of hypothalamic neurosecretory cells[4-6].
It is well known that the supraoptic nucleus (SON) plays a key role in regulation of osmotic pressure regulation. Increased Cx32 mRNA levels in rat supraoptic nuclei have been found in late pregnancy and during lactation[2]. But the ultrastructural characteristics of the gap junctions (GJs) between neurons and astrocytes in SON following osmotic stimulation are unknown. In the present study, the characteristics of ultrastructure at junction areas between the neurons and astrocytes were examined in rat SON following hyperosmotic stimulation by using an immuno-electron-microscopic technique, and a heterotypic Cx32/Cx43 GJs at junction areas between neurons and astrocytes was observed.
It is well known that carbenoxolone (CBX), a drug to treat gastric ulcer, could block information transmitting via GJs, but whether CBX affects action of GJs between the neurons and astrocytes in SON is not clear. Thus, we studied the effect of CBX on GJs and found that CBX inhibited the activity of neurons in SON rather than the activity of astrocytes.
MATERIALS AND METHODS
Animal model
Ten adult male Sprague-Dawley rats (250-300 g) were provided by the Laboratory Animal Center, Fourth Military Medical University (FMMU, Xi’an, China) and divided into experimental group and control group. The protocols used in animal study were approved by the FMMU Committee of Animal Use for Research and Education. Adequate measures were taken to minimize pains or discomforts for all experimental animals. The experimental animals were fed with 30 g/L NaCl solution, the control animals with fresh water. After 5 days, the animals were anesthetized (ip) and transcardially perfused with 500 mL solution of 0.1 mol/L PB (pH 7.4) containing 40 g/L paraformaldehyde and 2 g/L saturated picric acid for 0.5 hour. Hypothalamus including SON was then removed immediately and placed in 0.05 mol/L PB containing 200 g/L sucrose at 4 overnight.
Tissue preparation
Hypothalamus including SON was cut into 50 µm thick frontal sections on a vibratome (Microslicer DTK-100; Dosaka, Kyoto, Japan) and placed into 0.01 mol/L KPBS for 60 minutes. Subsequently, the sections were frozen in liquid nitrogen for enhancement of penetration of antibody. Then the sections were placed in 0.01 mol/L KPBS and divided randomly into three groups.
The sections in the first group were incubated in rabbit polyclonal antibody against GFAP (1:3 000, Dako) for 48 hours at 4 °C, and then in secondary goat anti-rabbit IgG (1:500, Sigma) and in ABC complex (1:500, Sigma) at room temperature for 2 hours. Finally, the sections were visualized with glucose oxidase-DAB-nikel as a chromogen.
The sections in the second group were incubated in rabbit anti-Cx43 antibody (Chemicon, CA) for 48 hours at 4 °C, and then processed according to the methods mentioned above. After washed, the sections were again incubated with monoclonal antibody against Cx32 (Chemicon, CA) and labeled with 5nm gold particles.
The sections in the third group were used as control. After washed with 0.01 mol/L PBS, the sections were post-fixed with 10 g/L OsO4 in PB for 45 minutes, dehydrated through a graded ethanol and propylene oxide, flat-embedded with Epon 812. The sections were examined with a light microscope and the regions containing GFAP -like immunoreactive (-LI) cells or Cx-32- and Cx43- LI cells were investigated under an electron microscope. Small pieces from tissue were sampled from SON and re-embedded in beam capsules. Tissue samples from the selected regions were cut into sections on an ultramicrotome (Reichert – Nissei S; Leica, Vienna, Austria), and prepared for the study with electron microscope (H-7100; Hitachi, Tokyo, Japan).
CBX blockade
Twenty-one adult male SD rats were divided into three groups. The rats in control group (n = 5) were intravenously injected with 9 g/L NaCl (1 mL). The rats in hyperosmotic group (n = 10) were intraveneously injected with 90 g/L NaCl (1 mL). The rats in the third group rats (n = 6) were pre-injected with CBX (1.5 μg/g, 10 g/L) into the lateral ventricle and 2 hours latter, injected with 90g /L NaCl (1 mL) into the femoral vein. One hour after NaCl injection, all the rats in three groups were transcardially perfused. The brains were removed with the methods mentioned above. Then the hypothalamus including SON was cut into 30 µm thick frontal sections on a cryostat (Cryostal; Leitz, Wetzal, Germany).
The sections from each rat were randomly divided into three sets. Two sets were processed for anti-Fos and anti-glial fibrillary acidic protein (GFAP) immunohistochemical staining respectively. Briefly, the sections were incubated with rabbit polyclonal antibodies anti-Fos (1:3 000; Santa Cruz), and GFAP (1:3 000; Dako) at 4 °C for 48 hours, and then in goat anti-rabbit IgG (1:500, Sigma) and ABC complex (1:500, Sigma) at room temperature for 2 hours. Nickel-intensified DAB reaction was used to detect peroxidase. The other set group of sections was treated as control, and processed without primary antibodies and therefore no immunoreactivity was found.
RESULTS
The behaviors of control animals were normal, while experimental animals looked languid and emaciated.
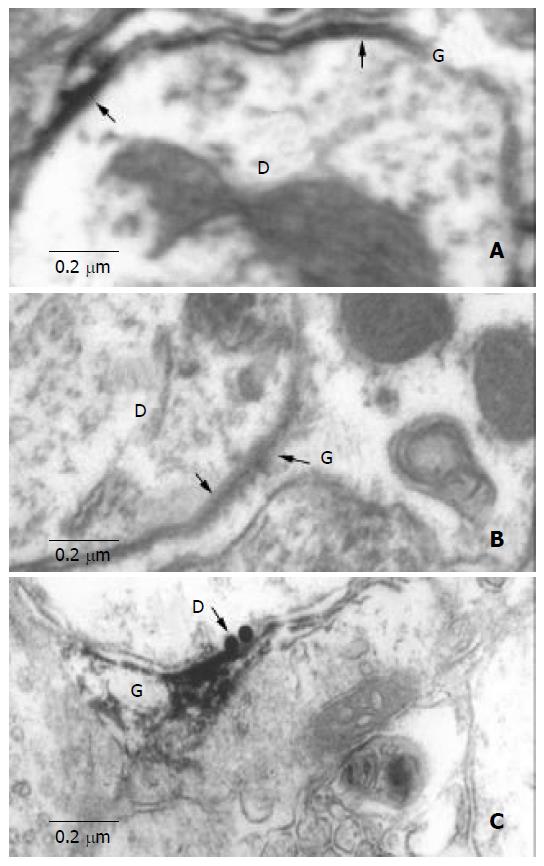
Puncta electron dense areas at the membrane of junction areas between neurons and astrocytes within SON were found on control sections. This structure consisted of astrocytic process on one side and neurons (cell body or dendrite) on the other side. It was characterized by thickened and dark stained membrane with a 2nm-cleft between them (Figure 1).
Figure 1 Electron microscopic observation of HGJ.
A: A dendrite (D) contacts with the process of ASs (G), the arrow indicates the thickened and dark stained cellmembrane, and there is a narrow cleft between them. B: Peroxidase labeling shows a dendrite (D) and a process of astrocyte (G). C: Located between the Cx32-LI dendrites (D) (5 nm black gold particles labeling, black arrow) and astrocytic process (G) (peroxidase labeling).
We also observed anti-GFAP-like immunoreactive (-LI) processes located within astrocyte side of the junction area between neurons and astrocytes (Figure 1B).
The results of anti-Cx32 and anti-Cx43 double immuno-electron-microscopic reaction indicated that Cx32-LI and Cx43-LI appeared in the neuron side and astrocyte side of the junction areas respectively (Figure 1C). We concluded that puncta electron dense areas at the junction areas might be the heterotypic Cx32/Cx43 gap junction that was called heterotypic gap junction (HGJ) in our study.
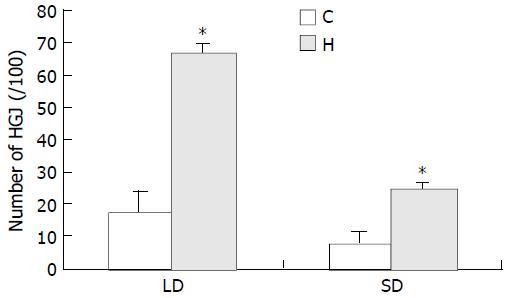
It is of interest to note that the number of HGJ in SON of the experimental group rats was significantly increased compared with that in control. We found that 17.43 per 100 large dendrites ( ≥ 1 μm) and 7.43 per 100 smaller dendrites ( < 1 μm) had specialized areas in SON of normal control rats, while in SON of experimental group rats, 66.75 per 100 large dendrites ( ≥ 1 μm) and 24.35 per 100 smaller dendrites ( < 1 μm) were found to bear HGJs. Statistical analysis suggested that there was a significant difference in the number of dendrites and axons bearing specialized areas between the control and experimental rats (Figure 2). It indicated that HGJs could sensitively respond to stimulation.
Figure 2 Histogram comparison between hyperosmotic group (H) and control group (C) demonstrating percentages of HGJ formed by processes of astrocytes with neuronal dendrites (LD: large dendrites, SD: small dendrites).
The percentage indicated the ratio of positive LD or SD bearing HGJ in the total number of LD or SD, respectively. Significant difference was seen be-tween the experimental group and control group (P < 0.001).
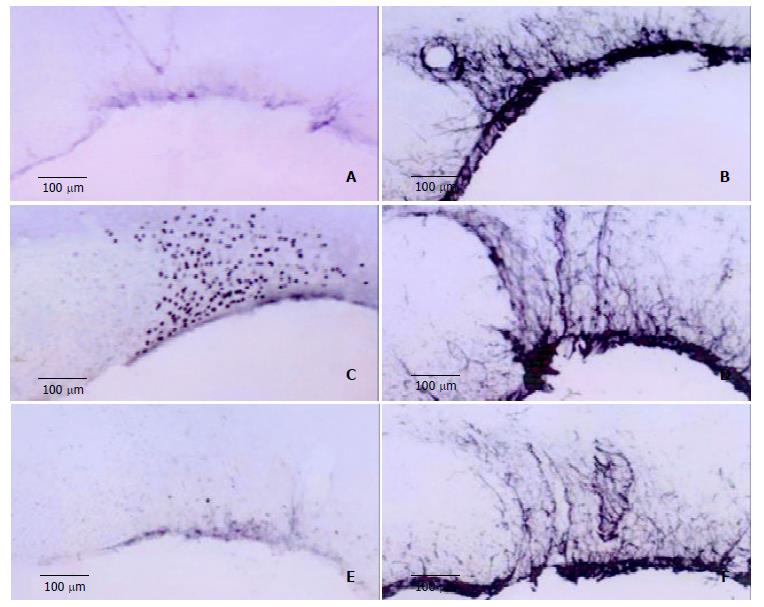
CBX inhibited expression of Fos-LI neurons. In control group, Fos-LI neurons in SON were not found and a few GFAP-LI astrocytes were observed. In hyperosmotic group, the number of Fos-LI neurons was significantly increased compared with that in control group. The number of GFAP-LI astrocytes was also slightly increased, and activated GFAP-LI astrocytes became hypertrophied and their processes became thickened. In the third group, the number of Fos-LI neurons in SON was decreased more significantly than that in hyperosmotic group, while GFAP-LI ASs did not differ remarkably (Figure 3).
Figure 3 A,B: Expression of Fos-LI neurons and GFAP-LI ASs in SON of control group rats.
C, D: Number of Fos-LI neurons and GFAP-LI ASs in hyperosmotic stimulation group compared to that of control group. E, F: Inhibited expression of Fos-LI neurons in CBX-injected rats, and uninhited GFAP-LI ASs.
DISCUSSION
It is well known that peripheral osmoreceptors are mainly localized in the mesenteric vasculature of the upper small intestine and the hepatic portal vein[3]. The osmotic, immune, and noxious information from these peripheral receptors could transmit to medullary nuclei (medullary visceral zone-MVZ that include nucleus of the tractus solitarius and ventrolateral medulla) via vagal nerve[4-8]. The neurons in medullary nuclei could relay information and reach hypothalamic neurosecretory cells of the supraoptic nucleus (SON) and paraventricullar nucleus (PVN)[4-6].
Our study indicated that astrocytes, as well as neurons, could sensitively respond to hyperosmotic stimuli induced by administration of 30 g/L NaCl solution orally or 90 g/L NaCl solution intravenously. Activated astrocytes appeared to be GFAP-LI positive, and activated neurons exhibited Fos-LI nuclei. The activated astrocytes have been found to be generally marked with GFAP, and neurons with Fos[9-12].
The discovery of intercommunication between neurons and astrocytes was an important advance in neurobiology in recent twenty years[13]. It has been that gap junction is an important channel of intercellular communication and also a direct link of the interiors of cells[14-16], and consists of connexins of the protein family[17,18]. However, about 15 types of Cx (followed by molecular mass designation) have been identified in mammals including the nervous and non-nervous systems[19,20]. At least 9 of the 15 types of Cx identified so far were expressed in CNS[21]. The same Cx types could be expressed in different cells and more than one Cx type could be expressed by the same cell. Nagy and Rash[18] reported that Cx30 was labeled by 5nm gold particles and Cx43 was labeled with immunoperoxidase, which was observed at the same astrocytic GJ in rat brain. Neurons and oligodendrocytes expressed Cx32, astrocytes expressed Cx43, and ependyma and leptomeningeal cells expressed both Cx26 and Cx43.
It is well known that GJs may occur between the same cell types such as neurons and glias, they may also occur between different cell types such as neuron and astrocyte, neuron and oligodendrocyte, and astrocyte and oligodendrocyte. GJs consisting of the same Cx protein have been termed as homotypic GJs[14-16], while that consisting of different Cxs at two sides of GJs in culture as heterotypic Cxs GJ[17,21]. It was reported that heterotypic Cx45/Cx43 GJs were observed in culture HeLa cells[11,22,23] and the oligo-astrocyte GJs in white matter arose by pairing astrocytic Cx43 with oligodendrocyttic Cx45[24]. Whether there were GJs between the neurons and astrocytes, some studies suggested that astrocytes might play a direct role in neuromodulation through GJs-mediated interaction between astrocytes and neurons[15,17]; Meanwhile, on the basis of fracture replica immunogold labeling, no evidence has been found for GJs between glial cells and neurons in the adult brain[19].
This study demonstrated that Cx32 and Cx43 appeared simultaneously at the neuronal side and astrocyte side, respectively, and formed the heterotypic Cx32/Cx43 gap junction. The special heterotypic Cx32/Cx43 gap junctions observed in this study had a narrow cleft (diameter: 2nm) between the neuronal and astrocytic membranes. This structure differed from the conventional gap junction, which has a seven-layer structure. It is of interest to note that the number of HGJs in SON of experimental group rats was significantly increased compared with that in control rats. In this study we also observed other types of ultrastructures, for example, synapses between neurons and astrocytes, tripartite synaptic structures and the gap junction between glial cells, but their number and structure did not show any significant change following stimulation (data not shown). It indicated that HGJs might be involved in signal communication of osmotic pressure regulation.
Results from this study indicated that the expression of Fos-LI neurons in experimental animals, which were injected CBX into the lateral ventricular, was markedly decreased compared with control animals. CBX, a glycyrrhetinic acid, is used to treat gastric ulcer by blocking information transmitting via gap junction. It was reported that CX cloud block GJs between neurons, and delay induction of epileptiform activity and reduces established epileptiform activity[25-27], and significantly decrease the spread of cell death induced by ischemia[28]. CX could also blocks GJs between glias[29,30] or between neurons and glias[15,31].
There has been debate on direction of intercommunication between the neurons and astrocytes coupling. Nedergaard[32] and Robinson et al.[33] described that unidirectional dye coupling from astrocytes to neurons and from astrocytes to oligodendrocytes occurred in intact neural tissues. Alvarez-Maubecin et al.[15] also suggested the existence of functional coupling between brainstem glia and noradrenergic neurons in slices taken from postnatal animals. But Rozental et al. and Carmignoto[15,34] considered that there was bidirectional signaling through gap junction between neurons and astrocytes. The results from the present research indicated that the expression of Fos-LI SON neurons induced by hyperosmotic stimulation could be inhibited with CBX, while the response of astrocytes to stimulation was not affected. It is suggested that the signaling via HGJs is unidirectional from astrocytes to neurons.