INTRODUCTION
The oral delivery of peptide, protein, vaccine and nucleic acid-based biotechnology products is the greatest challenge facing the drug delivery industry. Oral delivery is most attractive due to easy administration, leading to improved convenience and compliance to patients, thereby reducing the overall healthcare cost. Gene therapy will provide a huge new therapeutic opportunity, and has stimulated an interest in oral gene delivery. To date, various methods have been used for oral gene therapy, such as cationic lipids, recombinant viruses, recombinant live bacteria, polymers, and particle bombardment to buccal mucosa[1-3].
Chitosan is a natural biodegradable mucoadhesive polysaccharide derived from crustacean shells, and a biocompatible polymer that has been widely used in controlled drug delivery[4-9], and it may provide a less immunogenic and non-toxic carrier for successful oral delivery of plasmid DNA. Complex coacervates of DNA and chitosan could be used as a delivery vehicle in gene therapy and vaccine design[10-12], and have been shown to increase transcellular and paracellular transport of macromolecules across intestinal epithelial monolayers[13-15], further indicative of its potential in oral gene delivery.
Erythropoietin is a glycoprotein, which stimulates red blood cell production. It is produced in the kidney and stimulates the division and differentiation of committed erythroid progenitors in the bone marrow. When the kidney function decreases, anemia or low red blood cells are developed. Erythropoietin is used in patients with anemia associated with chronic renal failure, and in cancer patients for stimulation of erythropoiesis during autologous transfusion. Erythropoietin is also a good reporter gene in gene therapy study in vivo, because obvious biological effect can be observed even in a low dose of it. Hormones of therapeutic interest like growth hormone and erythropoietin require a tight adjustment of dose delivery to prevent adverse effects. Since most of the physiological regulatory processes are difficult to transfer to engineered cells, transgene expression must rely on artificial regulatory systems. Artificial inducible expression systems use transcriptional stimulation by chimeric transactivating factors, the activity of which can be controlled by drugs such as tetracycline derivatives[16], mifepristone[17], ecdysone[18], or rapamycine[19]. Heard observed that retrovirus-engineered myoblasts expressing rtTA and the chimeric transactivator conferring doxycycline-inducible gene expression, could be stably engrafted in mice, thus allowing the long-term control of Epo secretion in vivo[20]. Here we reported the oral gene therapy using chitosan–DNA nanoparticles carrying murine Epo (mEpo driven by tetO-CMV and rtTA) and LacZ, and demonstrated their efficacy in delivering genes to enterocytes.
MATERIALS AND METHODS
Materials
Chitosan (molecular weight, about 300 000Da) was supplied by Shandong Luneng Chemical Company. Plasmid pCMVβ was purchased from Clontech. mEpo was a gift from Jean Michel Heard (MD, Laboratoire Re´trovirus et Transfert Ge´ne´tique, Institut Pasteur, Paris, France). A 630 bp DNA fragment containing the murine Epo coding sequence was inserted between tetO-CMV promoter and 5’ end of the SV40 polyadenylation signal puHD10.3[21]. An expression cassette for the reverse transactivator (rtTA) chimeric protein[16] was inserted into the SV40 polyadenylation signal in reverse orientation. This cassette contains a 1 858 bp fragment of the MFG retroviral vector[22] encompassing the 5’ LTR and gag intronic sequences, followed by an 1 020 bp of the rtTA coding sequence.
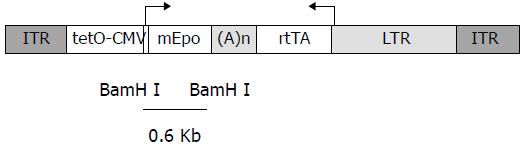
The construction was then constructed into the pSUB-201 AAV vector plasmid, giving rise to pAAV-ET, with a total length of 5 017 bp (Figure 1). Plasmid was purified by CsCl super centrifugation.
Figure 1 Structure of rAAV-ET vector.
ITR: AAV-2 inverted terminal repeats, tetO-CMV: tetracycline-inducible promoter including seven repeats of the tetracycline operator inserted upstream of the CMV minimal promoter, mEpo: murine eryth-ropoietin cDNA, (A)n: SV4O bidirectional polyadenylation signal, rtTA: coding sequences for the tetracycline reverse transactivator, LTR: long terminal repeat of the MFG retrovirus construct. The BamHI fragment used as an Epo-specific probe is indicated.
Nanoparticle formulation
Nanoparticles were made by complex coacervation of chitosan and DNA as reported[12]. Plasmid (10 μg) was added to 100 μl of 50 mM sodium sulfate and heated to 55 °C. Chitosan (pH 5.7, 0.02% in a 25 mM sodium acetate-acetic acid buffer) was also heated to 55 and 100 μl of chitosan was added to the DNA– sodium sulfate solution while samples were vortexed at a high speed for 20 s. Complex particles were examined immediately and stored at room temperature.
Measurement of nanoparticle size and morphology
Transmission electron microscopy (TEM, Hitachi HU-11B) was used to determine the particle size and morphology. A drop of particle dispersion was placed on a carbon- coated copper grid, and the particle size was determined from the micrographs.
DNase degradation test
Agarose gel electrophoresis was performed in a 1% (w:v) gel, ethidium bromide included for visualization, for 2 h at 60V. To assess the efficiency of encapsulation and stability against nuclease digestion, uncomplexed plasmid DNA (1 μg) and chitosan-DNA complex (containing 1 μg plasmid) were incubated with 1 mU DNaseI per mg of DNA for 1 h at 37 °C. Adding EDTA stopped the reaction. Then the undegraded (1 μg) and degraded plasmid, undegraded and degraded chitosan-DNA complex, were subjected to agarose gel electrophoresis as described above.
Gene expression and animal experiments
ICR mice (4-week-old, purchased from BK Company, Shanghai) were fed with either chitosan–DNA nanoparticles containing the LacZ gene (pCMVβ, 50 μg/mice) or ‘naked’ plasmid DNA (pCMVβ), using animal feeding needles. Three days later, the mice were killed, with their stomachs and small intestines surgically removed. The whole tissues were stained with 4-chloro-5-bromo-3-indolyl-β-galactoside (X-Gal) according to standard protocols. After stained overnight in a humidified chamber, the tissues were photographed by a digital camera (Nikon CoolPIX995). The pictures were transferred into a computer and adjusted for equal brightness and contrast using Adobe Photoshop.
ICR mice were fed every week with either chitosan–DNA nanoparticles containing the mEpo gene (50 µg/mice) or ‘naked’ plasmid DNA using animal feeding needles. Doxycycline-HCl (Sigma, Saint-Quentin Fallavier, France) was dissolved in drinking water to a final concentration of 200 µg/mL with 5% sucrose. No obvious side effect was observed in animals. Hematocrit was measured every two days by collecting 40 μL of blood via retro-orbital puncture before and after feeding.
Statistical analysis
Results of quantitative data in hematocrit analysis were expressed as mean ± SD, statistical differences between groups were tested with F-test, and the significant level was defined as a P < 0.01. The data were analyzed by SPSS statistical software.
RESULTS
Nanoparticle synthesis and characterization
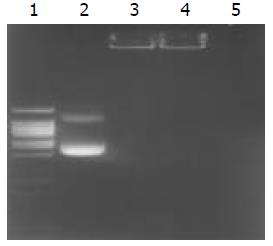
We synthesized nanoparticles by complexing high-molecular-weight (about 300 000Da) chitosan with plasmid DNA, and obtained the particles by adding 0.02% chitosan, pH 5.7, at 55 °C to plasmid DNA (50 μg/ml in 50 mM sodium sulfate) during high-speed vortexing. Transmission electron microscopy showed that freshly prepared particles were approximately 70-150 nm in size and fairly spherical (Figure 2). The encapsulation coefficiency was higher than 98 %, and the complex could efficiently protect the plasmid from DNase degradation (Figure 3).
Figure 2 Measurement of microsphere size and morphology.
Transmission electron micrographs of chitosan–DNA nanoparticles, and scale bar represents 100 nm.
Figure 3 Determination of encapsulation coefficiency, and DNase degradation test.
Line 1. DNA ladder, Line2. undegraded pCMVβ (1 μg), Line 3. undegraded chitosan-DNA complex (containing plasmid 1 μg), Line 4. degraded chitosan-DNA com-plex (containing plasmid 1 μg), Line 5. degraded plasmid (1 μg).
Gene expression studies
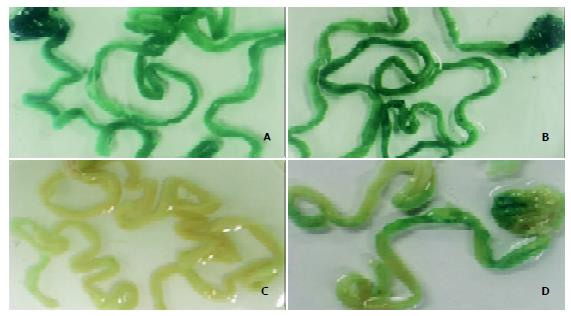
To assess the expression and distribution of transduced genes after oral DNA delivery, we fed ICR mice with either chitosan– DNA nanoparticles containing the LacZ gene (pCMVβ) or ‘naked’ plasmid DNA (pCMVβ), and then determined the expression of bacterial β-galacotosidase (LacZ) in the stomach and small intestine 3 days after oral administration of DNA (Figures 4A-D). Most of the whole small intestines and stomachs were stained. Although naive mice and those fed with ‘naked’ DNA showed some background staining, mice fed nanoparticles showed a higher level of gene expression both in stomachs and in small intestines.
Figure 4 β-galactosidase expression in mouse stomach and small intestine 3 days after oral delivery of DNA nanoparticles.
A-D: Whole tissue staining for LacZ, only stained sections are shown. The mice were fed with high-molecular-weight chitosan-pCMVβ nanospheres at a dose of 50 μg (A) or 100 μg (B) per mouse, PBS (C) or ‘naked’ DNA (pCMVβ, D).
mEpo expression and its physiological effect
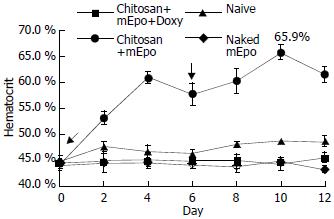
Hematocrit was measured every two days in mice that were fed with ‘naked’ mEpo DNA, chitosan-mEpo and doxycycline-HCl or not (Figure 5). To assess the basal Epo secretion level, three animals were fed with chitosan-mEpo and not treated with doxycycline, and their hematocrit remained 45%-48%. Doxycycline (200 µg/mL) was added to drinking water for naive (n = 4), ‘naked’ mEpo-fed mice (n = 5), and chitosan-mEpo-fed mice (n = 9). Though hematocrit was not modified in naive and ‘naked’ mEpo-fed mice, a rapid increase was observed during the first 4 days of treatment in chitosan-mEpo-dox-fed animals, reaching 60.9 ± 1.2%, and sustained for a week. The second feed (6 days after the first feed) seemed to promote a second hematocrit increase in chitosan-mEpo-dox-fed animals, reaching 65.9 ± 1.4%, while the second hematocrit increase did not appear in the ‘naked’ mEpo-second-fed mice (Figure 5). Statistical analysis showed the hematocrit level in chitosan-mEpo-dox-fed group was significantly higher than that in other groups (F = 184.1, P < 0.01), while there were no significant difference between the ‘naked’ mEpo-fed group and naïve group (F = 0.3, P > 0.01). These results indicated that oral administration of chitosan-mEpo induced repetitively robust Epo secretion in doxycycline- treated mice.
Figure 5 mEpo expression and its physiological effect test.
He-matocrit was measured every two days in mice fed with (●) chitosan-mEpo and doxycycline (200 µg/mL), n = 9; (▲) chitosan-mEpo alone, n = 3; (◆) doxycycline (200 µg/mL) alone, n = 4; (■) naked mEpo and doxycycline (200 µg/mL), n = 5.↓indicating the mice fed with ‘naked’ mEpo DNA or chitosan-Epo.
DISCUSSION
In 1998, Heard et al transfected erythropoietin in mice by intramuscular injection of an adeno-associated vector, and they observed the long-term, high-level and controlled expression of erythropoietin in mice[23]. Although viral gene delivery vectors yielded a high transfection efficiency over a wide range of cell targets[24-26], they presented major drawbacks, such as virally induced inflammatory responses and oncogenic effects. To circumvent these obstacles, gene delivery research also aimed at the development of non-viral gene delivery vectors. To date, there have been many available methods that are able to deliver plasmids to cells in vitro and in vivo, such as liposomes[27], cationic polymers[28], electro-gene-transfer[29], ultrasound[30] and hydrodynamic[31], but they have also many disadvantages, such as toxicity, relatively low transfection efficiency as compared to viral gene delivery vectors, tissue damage and difficulties of application in humans.
However, chitosan is a useful oral gene carrier because of its adhesive and transport properties in the gut, and has already been available in a pill form as an alternative therapy to reduce dietary fat and cholesterol absorption[32]. Recently, chitosan has been successfully used to deliver a reporter gene (encoding chloram-phenicol acetyl transferase) orally to enterocytes, Peyer’s patches and mesenteric lymph nodes[33]. Chitosan, when complexed with plasmid DNA, can form stable nanoparticles that are endocytosed by cells in the gastrointestinal tract. Further more, its safety and non-toxicity have been shown in animal models and humans[12,34,35], indication that chitosan-DNA nanoparticles may be a useful tool for gene therapy by oral administration.
Ion concentration, temperature, pH and the ratio of chitosan to DNA (or N/P) were the four main factors that influenced the formulation of chitosan-DNA complex[36]. The conditions we used above, could make the complex smaller, more stable, and easier to be absorbed. The zeta potential was approximately + 10 mV at pH 5.7 and close to neutral at pH 7. Thus, the particles might be positively charged at gastric and early duodenal pH but neutral thereafter at more physiological or alkaline pH. Since pH influences the formulation and stability of the complex, the buffer that dilutes the complex is crucial in the test. The 25 mM sodium acetate-acetic acid buffer (pH 5.7) is able to partially neutralize the alkaline condition in small intestine, makeing the complex infect more enterocytes than other buffers (PBS, Ringer’s, buffer saline). The long-term expression and the expression level still need to be improved, such as to increase the complex’s absorptivity and to resist expression silence etc.
Further more, as chitosan is a mucoadhesive polymer[37-39], such DNA nanoparticles might adhere to gastrointestinal epithelia, be transported across the mucosal boundary by M cells and transfect epithelial and/or immune cells in the gut associated lymphoid tissues either directly or through ‘antigen transfer’, indicating that it may generate protective mucosal immune responses.