Published online Oct 1, 1995. doi: 10.3748/wjg.v1.i1.43
Revised: June 20, 1995
Accepted: August 20, 1995
Published online: October 1, 1995
AIM: Recently, silver-stained nucleolar organizing regions (AgNOR) analysis has been used as a criterion for tumor diagnosis and research. The purpose of this study was to investigate the prognostic value of AgNOR analysis in colorectal carcinomas.
METHODS: The silver staining technique was applied to paraffin embedded tumor tissue sections from 114 patients with colorectal carcinoma. The number, morphology, size, and distribution of AgNOR were counted and analyzed.
RESULTS: (1) The number of AgNOR in patients who died within 5 year of carcinoma diagnosis (x-± s: 8.8 ± 2.3 per nucleus, n = 27) was significantly higher than that in those who survived beyond 5 year (6.3 ± 1.8, n = 30, p < 0.001). The number of large sized (> 2 μm) and small sized (< 1 μm) AgNOR was significantly higher in patients who died (x-± s: 85.9 ± 20.7, 661.7 ± 250.5 in 100 nuclei) than in those who survived (71.7 ± 27.0, 398.3 ± 225.4, p = 0.04, 0.00 respectively). The concentrated type of distribution was significantly fewer in those who died (10.2%) than those who survived (31.4%, p = 0.00), whereas the mixed type of distribution was significantly greater in those who died (25.7%) than in those who survived (7.1%, p = 0.00). And (2) The number of AgNOR was also related to other factors that affected prognosis of colorectal carcinoma, such as age, histological type, depth of invasions, and metastasis to lymph nodes.
CONCLUSION: The AgNOR analysis is a novel and useful parameter for assessing the prognosis of colorectal carcinoma.
- Citation: Zhou ZF, Yuan SZ. Prognostic value of silver-stained nucleolar organizer regions in colorectal carcinoma. World J Gastroenterol 1995; 1(1): 43-47
- URL: https://www.wjgnet.com/1007-9327/full/v1/i1/43.htm
- DOI: https://dx.doi.org/10.3748/wjg.v1.i1.43
Nucleolar organizer regions (NOR) are markers of ribosomal DNA (rDNA) and rDNA transcription. It can provide information regarding gene regulation and the control of cell proliferation and differentiation[1] and can reflect the biological behavior of cancer cells. Recently, silver-stained nucleolar organizing regions (AgNOR) analysis has been applied to predict cancer prognosis; but the results have been controversial and no study to date has been performed in Chinese patients. In this study, we examined whether AgNOR quantitative analysis is predictive for colonic cancer prognosis and explored its relationship with other prognostic factors.
One hundred fourteen patients with colonic cancers were examined, of which 60 were male and 54 were female. Among them, 11 were < 29 year old (9.7%); 70 were 30-59 year old (61.4%); and 33 were over 60 (29.0%). In 44 cases, the cancer was located at the sigmoid colon; 18 at the ascending colon; 14 at both the descending colon and ileocecum; nine at the transverse colon; eight at the splenic flexure, and seven at the hepatic flexure of colon. In 57 cases, carcinomas invaded the serosa; 27 were beyond the serosa; 25 invaded deep smooth muscles; and 5 invaded the mucosa, submucosa, and shallow smooth muscles. Histological classification was graded in accordance with the diagnosis standards[2], as follows: 86 tubular adenomas (including 29 high, 36 moderate, and 21 low differentiations), six undifferentiated adenomas, five signet-ring cell adenomas, 12 mucoid adenomas, two papillary adenomas, and three polypoid adenomas. The tumors were classified according to malignant degree[3], as follows: 31 low malignant adenomas (including papillate and highly differentiated adenomas), 51 moderately malignant adenomas (including polypoid, moderately differentiated, and mucoid adenomas), 32 highly malignant adenomas (including low differentiated, undifferentiated, and signet ring adenomas). According to operation findings and pathohistological data from resected specimens, 56 cases had no metastasis, 47 had metastasis, and 11 cases were unclear in metastasis of lymphonodi. Among the cases of lymphonodi metastasis we studied, there were 39 cases of primary and metastatic cancers based on the AgNOR technique in identical slides. Fifty-seven cases were followed up for over 5 year , among whom 27 died and 30 survived. These preserved specimens of biopsy from patients with colonic cancer were obtained between 1984-1991 from the Department of Pathology, Sun Yat-sen Memorial Hospital, Sun Yat-Sen Medical University.
Preparation of AgNOR specimens. Tissues were fixed in 10% formalin solution and processed in paraffin wax. Each paraffin embedded block was cut into two 3 μm thick sections, with one routinely processed with hematoxylin and eosin stains and the other submitted to AgNOR staining according to Ploton’s modification one step method[4].
Quantitative analysis. Sections were examined in 20 ×, 40 ×, and 100 × immersion lenses and 100 × oil immersion lens. Fields were selected at random for analysis, and 100 cells were examined continuously. Each nucleus was examined in four respects, as follows: (1) Number of nucleoli: The number of nucleoli and the AgNOR number besides nucleoli were recorded. A nucleolus was defined as an AgNOR dot, and the mean number of AgNOR dots was calculated. (2) Shape: In each specimen, the number of regular type and abnormal type of AgNOR dots in 100 nuclei was counted in accordance with the following standards: 1. Regular type: the shape of the AgNOR dots was circular, and the rim was more or less smooth, 2. Abnormal type: the shape of the AgNOR dots was a bar shape rhombus or otherwise strange, with a diameter longer than 3 μm. (3) Size: The size of the AgNOR dot of each nucleolus was measured and classified into one of three groups: Large (2 μm), medium (1 μm), and small (0-1 μm), as measured with a C2 net type objective ruler (Shanghai Third Optical Instrument Factory, Shanghai, China). And (4) Distribution: In each specimen, the distribution of AgNOR dots in 100 nuclei was classified, as follows: 1. Gathered type: Regular dots were gathered at the center of nuclei. Generally, there were less than four dots per nucleus. 2. Scattered type: Irregular dots were scattered in the nuclei like satellites, and there were more than five dots per nucleus. 3. Mixed type: Nuclei had the characteristics of both gathered and scattered types.
Statistical analysis. Analysis of variance (ANOVA) or Kruskal and Wallis rank sum test (H test) were employed to detect the differences among groups. Meanwhile, Student’s t test or Wilcoxon rank sum test was performed to compare the differences between two groups. For paired designed data, either paired t test or Wilcoxon’s signed rank sum test was used.
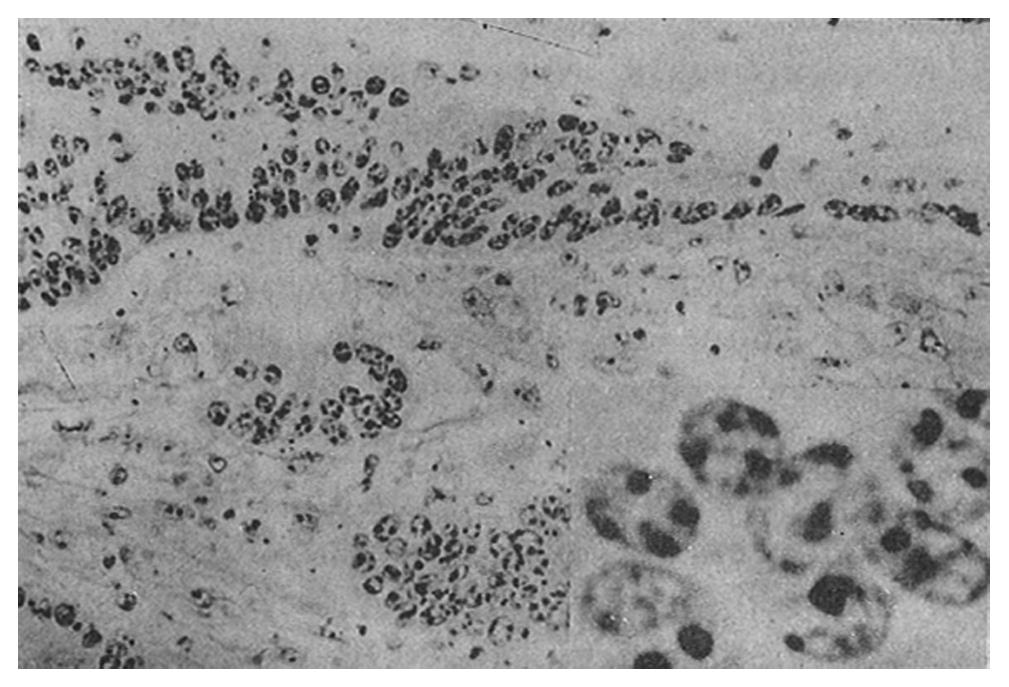
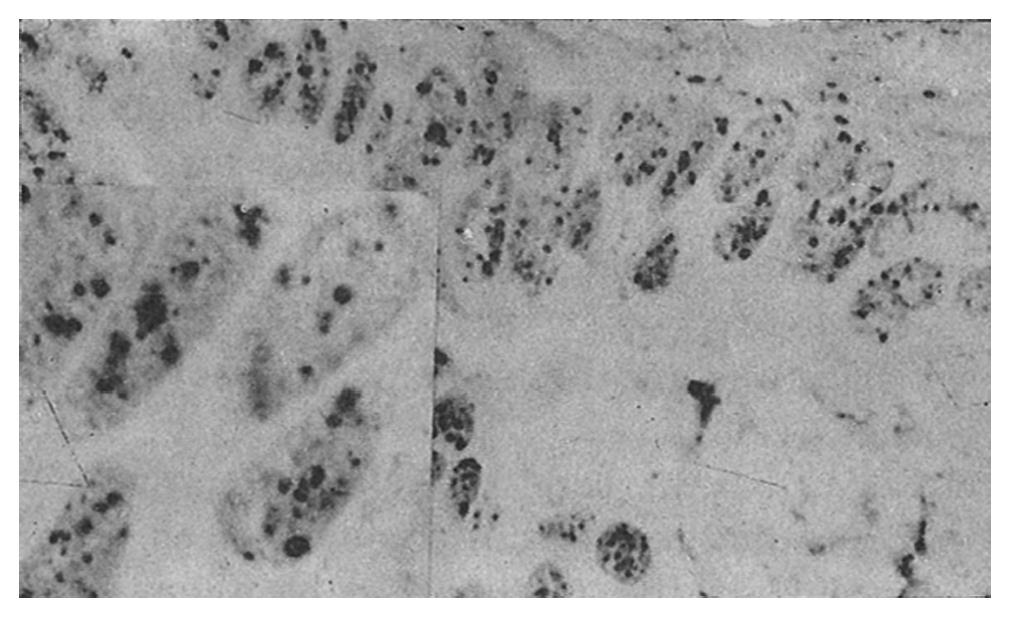
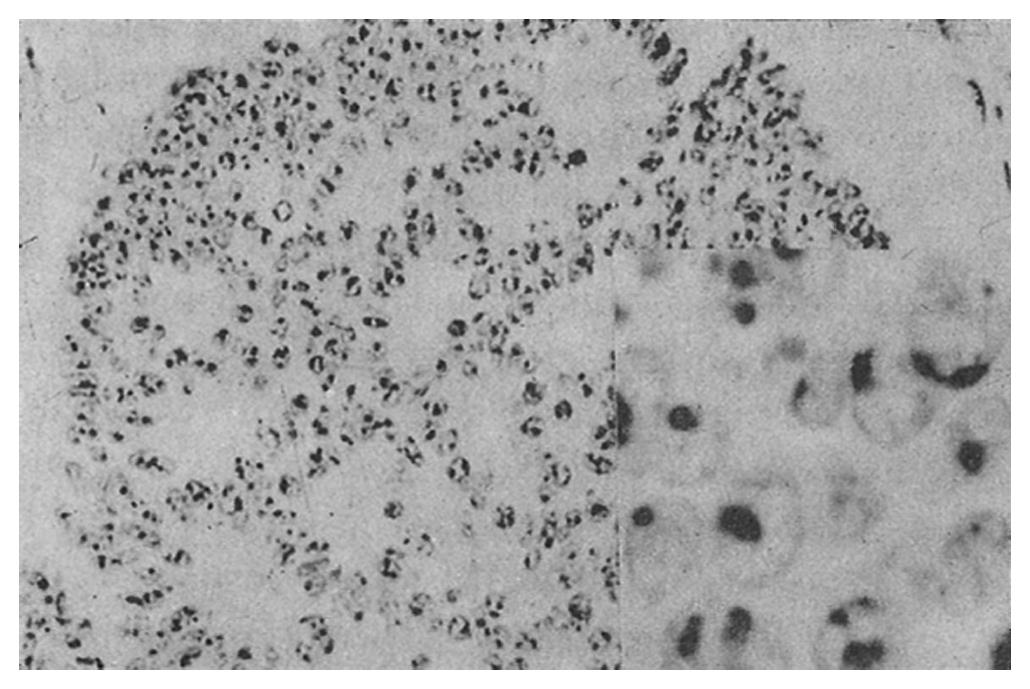
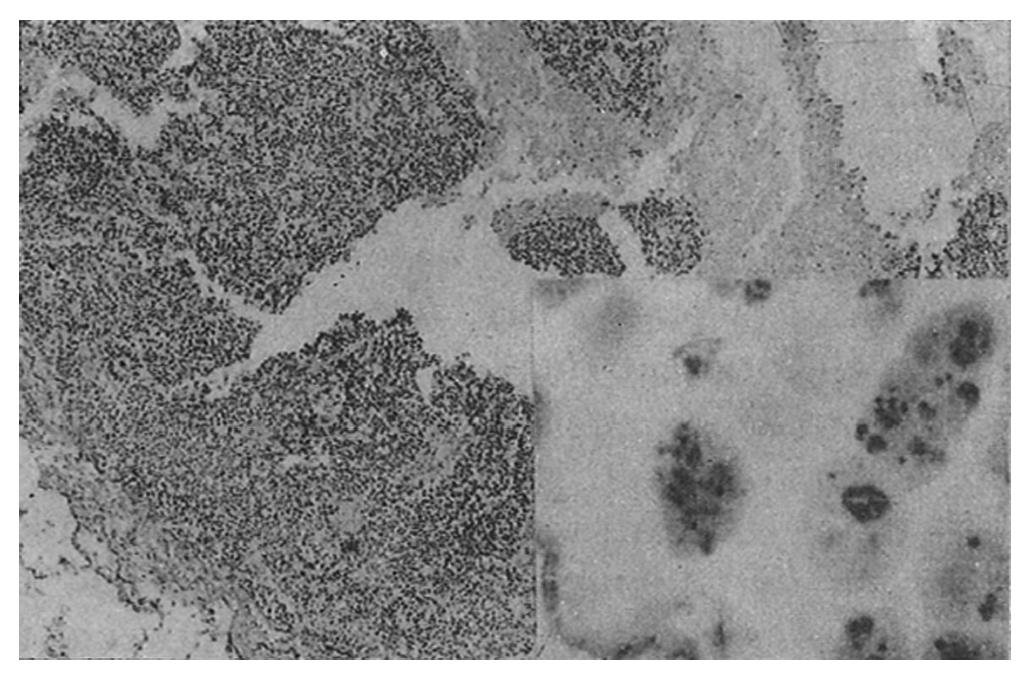
AgNOR was clearly recognized as black or brown dots in the nuclei or nucleoli after staining.
The mean number of AgNOR dots per nucleus was 7.1 ± 2.3 in the male colonic cancer group and 6.9 ± 2.1 dots in the female colonic cancer group (p > 0.05).
In the age groups < 29, 30-59, and over 60, the mean number of AgNOR was 8.5 ± 2.3, 7.1 ± 2.2, and 6.4 ± 1.8 dots per nucleus, respectively. The number of AgNOR in the < 29 age group was significantly higher than that in the 30-59 and the over 60 group (p < 0.05), and there was no difference between the latter two age groups (p > 0.05).
The mean number of AgNOR per nucleus was 6.6 ± 1.8 at the sigmoid colon, 7.6 ± 2.2 at the descending colon, 7.3 ± 3.0 at the splenic flexure of the colon, 7.0 ± 2.4 at the transverse of the colon, 6.7 ± 1.3 at the hepatic flexure of colon, 6.7 ± 2.1 at the ascending colon, and 8.5 ± 2.9 at the ileocecum. There were no significant differences among these groups.
The mean number of AgNOR of the colonic cancers that had invaded different layers was as follows: mucosa 5.6 ± 0.6, submucosa and shallow smooth muscle, 5.3 ± 0.9, deep smooth muscle, 7.4 ± 1.8, serosa, and 8.2 ± 2.9 in outer serosa. The number of AgNOR in cancers that had invaded the mucosa and submucosa and shallow smooth muscles was less than that in cancers that had invaded the serosa and outer serosa (p < 0.05). The mean number of AgNOR in the deep smooth muscle group was significantly different from that in the serosa and outer serosa groups (p = 0.00 for both), but there was no difference among other groups (p > 0.05).
Different types of colonic cancers: The mean number of AgNOR per nucleus was 6.6 ± 1.9 in tubular adenomas, 6.5 ± 0.9 in mucous adenomas, 11.9 ± 1.3 in undifferentiated adenomas, 10.5 ± 1.1 in signet ring cell adenomas, 5.7 ± 0.5 in polypous adenomas, and 6.3 ± 0.2 in papillate adenomas. The AgNOR number per nucleus in tubular adenomas was significantly different from that in signet ring cell adenomas and undifferentiated adenomas (both p < 0.01). In addition, the AgNOR number for mucosa adenomas was significantly different from both signet-ring cell and undifferentiated adenomas. No differences were detected among the other groups. Regarding different degrees of malignancy of colonic cancer, the mean number of AgNOR per nucleus was 5.2 ± 0.9 in the low malignancy group, 6.5 ± 1.2 in the moderate malignancy group, and 9.3 ± 2.5 in the high malignancy group (p < 0.01 for all).
In the metastasis of lymphonodi positive group, the number of AgNOR per nucleus was more than that in the lymphonodi metastasis negative group (7.6 ± 2.1 vs 6.4 ± 2.0 p = 0.00), and the AgNOR of the lymphonodi metastatic cancers was more than those of cancers in situ (8.1 ± 2.1 vs 7.3 ± 1.9, p = 0.00).
The mean number of AgNOR in those who died (8.8 ± 2.3) was significantly higher than that in those who survived (p = 0.00), and there was no overlap between the range of AgNOR number in these two groups, although the histological type was similar. The size and distribution of AgNOR, but not the shape, were significantly different between the two groups (Table 1, Table 2, Table 3, Figure 1, Figure 2, Figure 3, Figure 4).
| Histologic types | AgNOR numbers/nucleus | |||
| n | Survived group | n | Died group | |
| Tubular adenoma | ||||
| highly differentiated | 7 | 4.64 ± 0.23 | 8 | 6.26 ± 0.72 |
| 4.17-4.85 | 5.60-7.38 | |||
| moderately differentiated | 11 | 5.94 ± 0.92 | 7 | 8.44 ± 0.84 |
| 4.33-7.10 | 7.47-9.55 | |||
| low differentiated | 4 | 7.65 ± 0.74 | 6 | 11.23 ± 0.74 |
| 6.72-8.37 | 10.89-12.71 | |||
| Mucous adenoma | 5 | 6.33 ± 0.66 | 3 | 7.75 ± 0.39 |
| 5.70-7.29 | 7.42-7.94 | |||
| Other types1 | 3 | 9.64 ± 3.51 | 3 | 12.20 ± 0.39 |
| 6.02-11.02 | 11.88-12.63 | |||
| Total2 | 30 | 6.29 ± 1.83 | 27 | 8.76 ± 2.29 |
| 4.17-11.02 | 5.60-12.63 | |||
In recent years, there have been a number of substantial studies on the relationship between AgNOR and the prognosis of various cancers. Several studies on cancers of the digestive system have suggested that the number of AgNOR in patients who died was significantly higher than those who survive[5], and that the number of AgNOR was increased in the cases of invasion and metastasis of cancer cells[6]. Griffiths et al[7] evaluated 100 cases of rectum adenomas and found no relationship between AgNOR number and prognosis, cell proliferation, and cell DNA ploidy. Liu et al[8] found that the number of AgNOR was significantly different between patients who died and those who survived non-Hodgkin's lymphoma, but there was some overlap between the two groups in a few cases. Eusebi et al[9] found that the mean area of AgNOR from short term (< 34 mo) breast cancer survivors was larger than that of long term (> 3 year) survivors, with no overlap observed between the two groups. Therefore, the utility of AgNOR in predicting cancer prognosis is controversial.
In this study we used AgNOR quantitative analysis to assess the prognosis of colonic cancer. We found that the relationship between AgNOR and clinical prognosis is of value in clinical practice, as the number of AgNOR in the group of patients who died was significantly higher than that in the group that survived. In addition, the number of large and small dots increased; the distance between two polarities was increased (with the increase of small dots being most significant); and the distribution of gathered type decreased while the distribution of mixed type increased in the died group compared to the survived group. These results suggested that not only were the number of AgNOR different between the two groups but also their size and distribution. In addition, there was no overlap in the range of AgNOR numbers between the died and the survived groups, although the histological type was identical. These results were consistent with Eusebi's report. Moreover, we identified a relationship between AgNOR and correlative factors of colonic cancer prognosis. Some studies have suggested that the histological type of colonic cancer played a role in determining survival rate. Our data showed that the number of AgNOR was significantly different in different histological types of colonic cancers and that the number of AgNOR increased with the degree of malignancy. These results indicated that quantitative analysis of AgNOR could reflect cell proliferation, differentiation, and degree of malignancy and that clinical course process correlated well with survival.
Depth of invasion is one of the most important factors in colonic cancer prognosis, and prognosis was significantly different between the groups with shallow invasion and those with deep muscle, serosal, or beyond serosal invasion. Our study showed that the number of AgNOR per nucleus was significantly higher in the latter than in the former. These results also suggested that the number of AgNOR is correlated with the prognosis.
Lymph node metastasis is an important factor in the prognosis of colonic cancers. Bockmuhl et al[10] found that the number of AgNOR in breast cancer with lymph node metastasis was higher than that in breast cancer without lymph node metastasis. Kakeji et al[11] also found that the number of AgNOR was increased in gastric cancer when lymph node metastasis occurred. Our study showed that the number of AgNOR in the lymph node metastasis positive group was higher than that in the lymph node metastasis negative group. The increase of AgNOR number in infiltrative and metastatic cancer cells suggested that those cells were more biologically active and that rRNA gene duplication and transcription activity was much greater. Therefore, AgNOR number contributed to the prediction of cancer cell invasion, metastasis, and relapse after surgery. We also found that AgNOR number of lymph node metastatic cancer was higher than that of primary colonic cancer. The results were consistent with those previously reported by Ohno et al[12] in lung cancer with metastasis of cartilaginous sarcoma. The phenomenon might correlate with the aberrance of cancer. More efforts are needed to explore the mechanism in colonic cancers. The prognosis of cancers located at the rectum was poorer than that located at the colon, and the prognosis of cancers in the ileocecum and ascending colon was better than that in other parts of the colon. No difference among other sites of the colon was found[3]. However, our study showed that the number of AgNOR was not different in any location of colonic cancers, suggesting that the number of AgNOR was not correlated with the site of colonic cancer. Regarding age, the prognosis in the group below 30 year old was poorer than that in the 30-59 year and over 60 year groups[3]. Our study showed that in the below 30 year group, the number of AgNOR was higher than that in 30-59 year and over 60 year groups, suggesting that the number of AgNOR was increased in young people who had poorer prognosis.
AgNOR is a marker of cell proliferation and rRNA transcription. Therefore, cancers with a higher number of AgNOR have more active cell proliferation. These cancers are exuberant, with more biological activity. The patient's condition advanced faster, and their prognosis was worse. In contrast, in those with cancer with a lower AgNOR number, the proliferation of cells was relatively slow with good biological activity. The patients’ condition was stable, and the prognosis of patients was good. Moran et al[5]studied the prognosis of advanced colonic cancers and found that the traditional clinical and pathological indices were too difficult to use in the prediction of colon cancer prognosis. However, AgNOR were considered a reliable prognostic index. Ofner et al[13]studied AgNOR and several other prognostic indices of colonic cancer in post-operative patients and found that the value of AgNOR for predicting the prognosis of colonic cancers was more reliable and more accurate than that of the World Health Organization (WHO) classification system (UIC dividing terms, Jass classification and Duke's classification). Our study showed that AgNOR correlated well with clinical prognosis, age, histological types, the degrees of invasion, and lymphonodi metastasis. Between the died and survived groups with identical histological types, no overlap was observed. As shown in the present study, AgNOR quantitative analysis is a new, useful, and reliable index for predicting the prognosis of colonic cancers. In particular, it can be used for pathologic diagnoses before and after operation of colonic cancers. This analysis can also be used to direct treatment and to predict the metastasis and relapse of cancers. Taken together, our data suggest that AgNOR quantitative analysis has broad prospects for use in clinical practice.
This work was previously presented at the 2nd International Conference of Gastroenteropathy in (Chengdu), 1993.
Original title: China National Journal of New Gastroenterology (1995-1997) renamed World Journal of Gastroenterology (1998-).
S- Editor: Filipodia L- Editor: Jennifer E- Editor: Zhang FF
| 1. | Zhuang YH, Wang RN, Hu DY. The measurement of argyrophil protein of nucleolar organizer regions (AgNORs) and the role of AgNORs, in the study of digestive tumor. Foreign Medicine (Bromch of Digestive). 1989;9:82-84. |
| 2. | The Tumor Prevention Office of China. The tumor prevention association of China. Diagnosis and treatment rules of common cancer in China. Section 3 Large Intestine Cancer. First Ed, Beijing: The People’s Health Publishing House 1990; 11-20. |
| 3. | He MT. Current progress of pathology study on large intestine cancer. Linchuang Yu Shiyan Binglixue Zazhi. 1985;2:50-54. |
| 4. | Ploton D, Menager M, Jeannesson P, Himber G, Pigeon F, Adnet JJ. Improvement in the staining and in the visualization of the argyrophilic proteins of the nucleolar organizer region at the optical level. Histochem J. 1986;18:5-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 698] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 5. | Moran K, Cooke T, Forster G, Gillen P, Sheehan S, Dervan P, Fitzpatrick JM. Prognostic value of nucleolar organizer regions and ploidy values in advanced colorectal cancer. Br J Surg. 1989;76:1152-1155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Chen L, Xiao YF, Eu KR. Value of nucleolar organizer regiona2associated protein (AgNOR) in gastric lesions. Tianjin Yiyao. 1991;8:478-480. |
| 7. | Griffiths AP, Butler CW, Roberts P, Dixon MF, Quirke P. Silver-stained structures (AgNORs), their dependence on tissue fixation and absence of prognostic relevance in rectal adenocarcinoma. J Pathol. 1989;159:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Lln JQ, Zhang SL, Li SY. AgNOR in nona-Hodgkin’s lymphomasa2Its prognostic importance and the value in histological classfication. Linchuang Yu Shiyan Binglixue Zazhi. 1991;7:192-197. |
| 9. | Eusebi V, Cattani MG, Lamovec J, Treré D, Ceccarelli C, Veronesi P, Clemente C, Derenzini M. Prognostic relevance of silver-stained nucleolar proteins in sarcomatoid carcinomas of the breast. Ultrastruct Pathol. 1991;15:203-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Bockmühl U, Theissig F, Dimmer V, Kunze KD. The impact of nucleolar organizer regions for the lymph node spread and prognosis of invasive ductal mammary carcinoma. Pathol Res Pract. 1991;187:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Kakeji Y, Korenaga D, Tsujitani S, Haraguchi M, Maehara Y, Sugimachi K. Predictive value of Ki-67 and argyrophilic nucleolar organizer region staining for lymph node metastasis in gastric cancer. Cancer Res. 1991;51:3503-3506. [PubMed] |
| 12. | Ohno T, Tanaka T, Takeuchi S, Matsunaga T, Mori H. Silver-stained nucleolar organizer proteins in chondrosarcoma. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Ofner D, Tötsch M, Sandbichler P, Hallbrucker C, Margreiter R, Mikuz G, Schmid KW. Silver stained nucleolar organizer region proteins (Ag-NORs) as a predictor of prognosis in colonic cancer. J Pathol. 1990;162:43-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 1.7] [Reference Citation Analysis (0)] |