Copyright
©The Author(s) 2003.
World J Gastroenterol. Oct 15, 2003; 9(10): 2164-2168
Published online Oct 15, 2003. doi: 10.3748/wjg.v9.i10.2164
Published online Oct 15, 2003. doi: 10.3748/wjg.v9.i10.2164
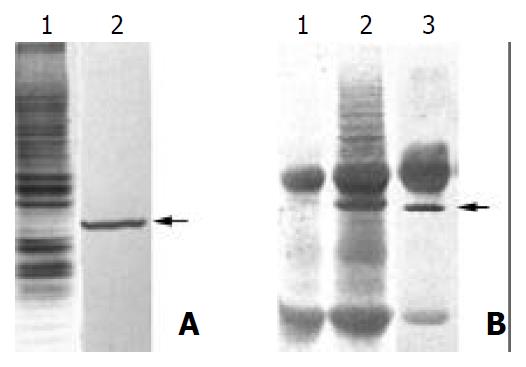
Figure 1 Purification of the antigen by MAb PD4.
(A) 1. SDS-PAGE and Coomassie blue staining of the membrane proteins extracted from MGC803 cells; 2. Western blot analysis of the membrane proteins extracted from MGC803 with MAb PD4, the arrow indicating the target protein of MAb PD4. (B) 1. SDS-PAGE analysis of immunoprecipited protein complex bound with normal mouse IgG. (B) 2. SDS-PAGE analysis of immunoprecipited protein complex bound with MAb PD4. (B) 3. Western blot analysis of immunoprecipited protein complex bound with MAb PD4, arrow indicating the target protein of MAb PD4.
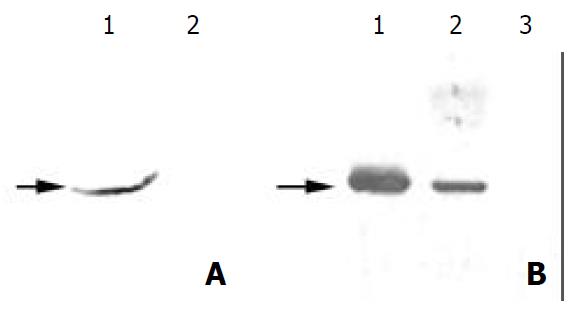
Figure 2 Further identification of the antigen from mycoplasma infection.
(A) 1. Western blot analysis of the total protein from Hela cells with MAb PD4, which was treated with cultured MGC803 medium. (A) 2. Western blot analysis of the total pro-tein from untreated HeLa cells with MAb PD4. (B) Western blot analysis of the total proteins from MGC803 cells treated with BM cyclin antibiotics differently. Lane 1: untreated, lane 2: treated for two weeks, and lane 3: treated for three weeks. As indicated by the arrow, the band reacted with MAb PD4 disappeared gradually following the treatment.
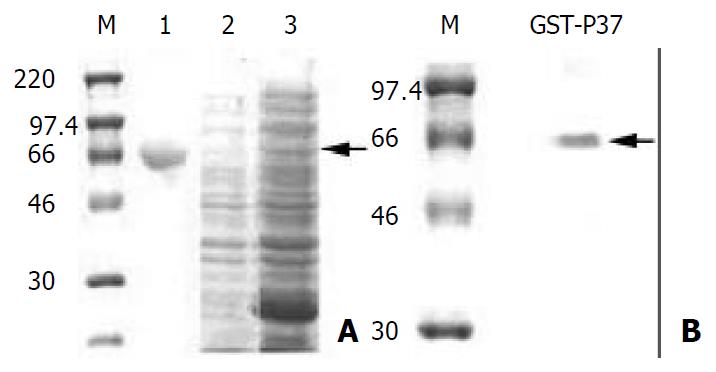
Figure 3 Analysis of GST-p37 with SDS-PAGE and Western blot.
(A) Coomassie blue staining of the SDS-PAGE gel. M pro-tein standards. lane 1: protein of BSA, lane 2: total proteins from un-induced bacteria, lane 3: total proteins from IPTG in-duced bacteria, the arrow indicating GST-p37 band. (B) West-ern blot analysis of purified protein with MAb PD4, the arrow indicating GST-p37 band.
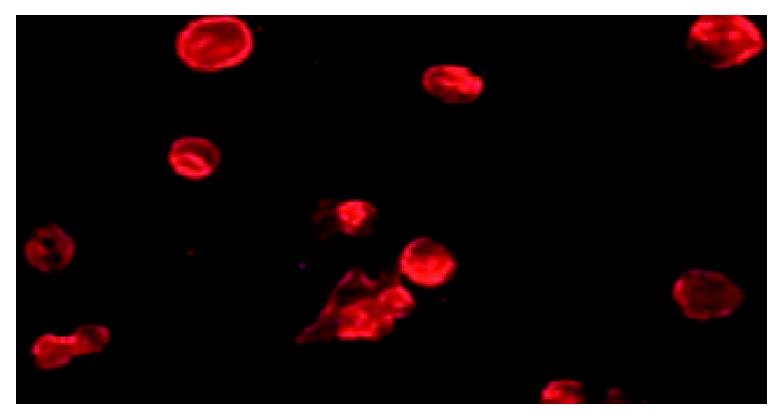
Figure 4 p37 binding AGS cells assay.
Observation of the immunofluorecence under microscopy, which showed GST-p37 protein binding to AGS cells, whereas GST protein has no such binding ability (data not shown).
-
Citation: Ning JY, Sun GX, Huang S, Ma H, An P, Meng L, Song SM, Wu J, Shou CC. Identification of antigens by monoclonal antibody PD4 and its expression in
Escherichia coli . World J Gastroenterol 2003; 9(10): 2164-2168 - URL: https://www.wjgnet.com/1007-9327/full/v9/i10/2164.htm
- DOI: https://dx.doi.org/10.3748/wjg.v9.i10.2164