Copyright
©The Author(s) 2002.
World J Gastroenterol. Jun 15, 2002; 8(3): 446-450
Published online Jun 15, 2002. doi: 10.3748/wjg.v8.i3.446
Published online Jun 15, 2002. doi: 10.3748/wjg.v8.i3.446
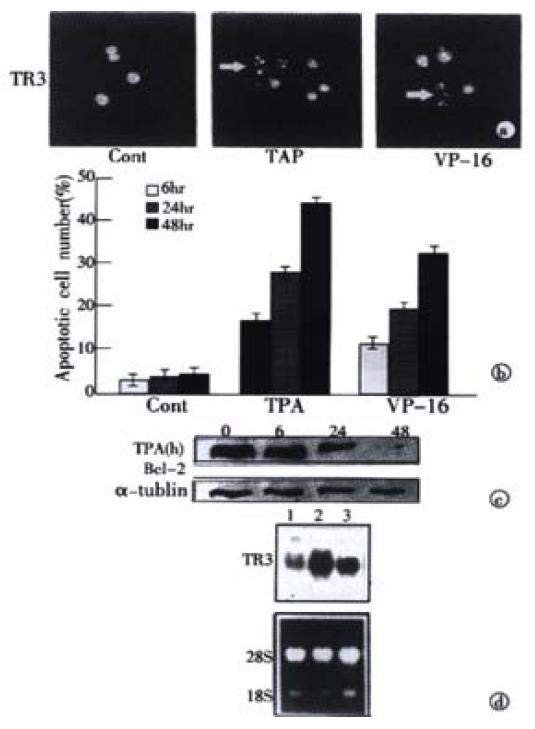
Figure 1 Induction of apoptosis and TR3 expression induced by TPA and VP-16 in MGC80-3 cells.
(A) Morphological analysis of apoptotic cells. Cells treated with TPA and VP-16 for 24 hr, and then stained with DAPI. Nuclear mor-phology was visualized under fluorescence microscope. (B) Measure of apoptotic index by counting 1000 cells stained with DAPI under fluorescence microscope. The data shown represents mean of three independent experiments (± SE). (C) Analysis of Bcl-2 protein expression. Cells were treated with TPA for indicated time, and Western blot was preformed as described in materials and methods. α-tubulin was used to quantify the amount of protein used in each lane. (D) Detection of TR3 mRNA expression. Cells were treated with TPA and VP-16 for 24 hr. Preparation of total RNA and Northern blot were carried out as described in materials and methods. 18S and 28S were shown to quantify the loading RNA. Lane 1: control; Lane 2: TPA treatment; Lane 3: VP-16 treatment.
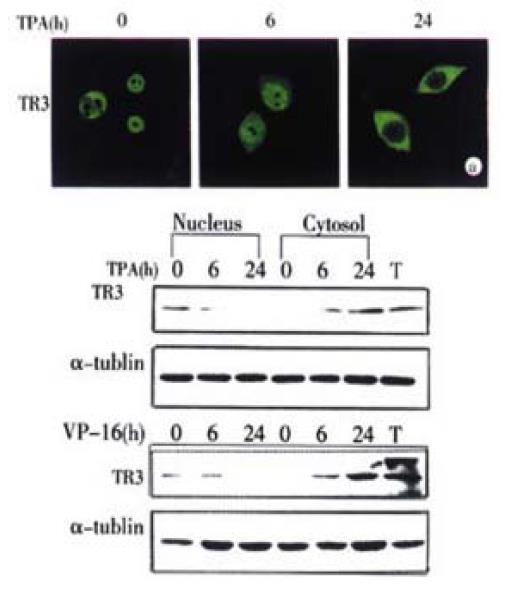
Figure 2 Translocation of TR3 protein from nucleus to cytosol in MGC80-3 cells.
The cells treated with TPA for indicated time. (A) Translocation of TR3 protein observed by laser-scanning confocal microscope. TR3 protein was immunostained with anti-TR3 antibody and corresponding FITC-conjugated secondary antibody. (B) Western blot showed the translocation of TR3 protein. Nuclear and cytosolic fractions were prepared as described in materials and methods. α-tubulin was shown to quantify the loading protein. T: total protein.
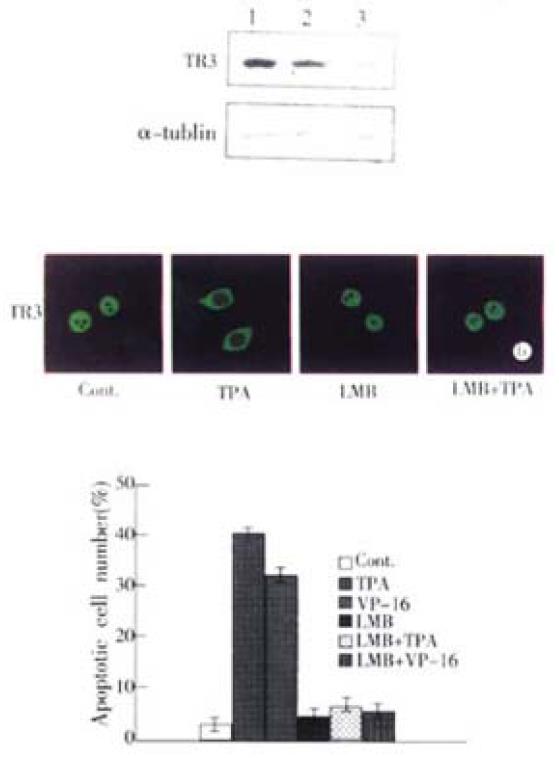
Figure 3 Inhibition of TR3 protein expression and its translocation in MGC80-3 cells.
(A) Repression of TR3 protein expression by transfection of antisense-TR3 expression vector into MGC80-3 cells. Endogenous TR3 pro-tein was determined by Western blot. Empty vector was also transfected into cells as a positive control. α-tubulin was shown to quantify the loading protein. Lane 1: protein from MGC80-3 cells; Lane 2: protein from the cells transfected with empty vector; Lane 3: protein from the cells transfected with antisense-TR3 expression vector. Inhibitory effect of LMB on TR3 protein translocation induced by TPA. The cells treated with different agents indicated as in the figure. The reaction with antibody was similar to the description in Figure. 2A. (C) Inhibitory effect of LMB on apoptosis induc-tion by TPA and VP-16. Cells treated with different agents indicated as in the figure. Apoptotic index was measured as described in Figure 1B.
- Citation: Liu S, Wu Q, Ye XF, Cai JH, Huang ZW, Su WJ. Induction of apoptosis by TPA and VP-16 is through translocation of TR3. World J Gastroenterol 2002; 8(3): 446-450
- URL: https://www.wjgnet.com/1007-9327/full/v8/i3/446.htm
- DOI: https://dx.doi.org/10.3748/wjg.v8.i3.446