Copyright
©The Author(s) 2001.
World J Gastroenterol. Apr 15, 2001; 7(2): 228-234
Published online Apr 15, 2001. doi: 10.3748/wjg.v7.i2.228
Published online Apr 15, 2001. doi: 10.3748/wjg.v7.i2.228
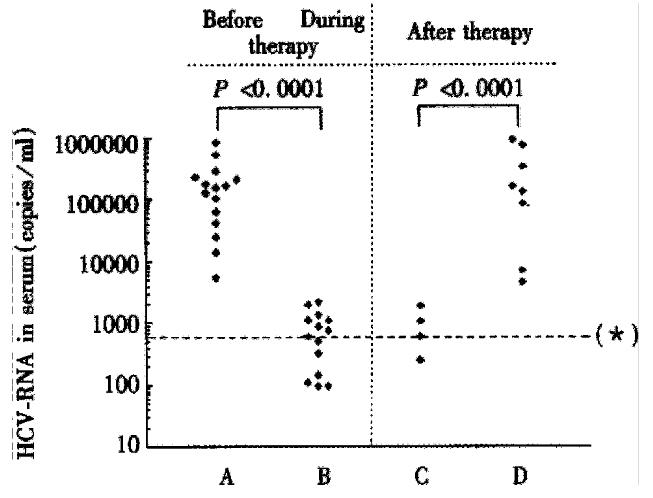
Figure 1 HCV-RNA concentration (copies/mL) in sera from patients with chronic HCV infection with regard to HCV-RNA detectability in PBMC before, during and after an IFN-α therapy.
The transition from detectable to non-detectable HCV-RNA in PBMC during drug therapy was associated with a strong and significantly decrease of serum HCV-RNA concentration. Vice versa, the transition from non-detectable to detectable HCV-RNA in PBMC, in the case of a breakthrough under therapy, or when therapy was stopped and a relapse was observed was associated with a marked and significantly increase of serum HCV-RNA concentration. Columns A and D: HCV-RNA concentration, if HCV-RNA in serum and in PBMC was detectable. Columns B and C: HCV-RNA concentration, if HCV-RNA in serum was detectable and in PBMC undetectable. (*) Test results less than 600 copies/mL are below the lower limit of quantification of the test and should be reported as “HCV detected, less than 600 copies/mL”.
- Citation: Meier V, Mihm S, Braun PW, Ramadori G. HCV-RNA positivity in peripheral blood mononuclear cells of patients with chronic HCV infection: does it really mean viral replication? World J Gastroenterol 2001; 7(2): 228-234
- URL: https://www.wjgnet.com/1007-9327/full/v7/i2/228.htm
- DOI: https://dx.doi.org/10.3748/wjg.v7.i2.228