Copyright
©The Author(s) 1999.
World J Gastroenterol. Oct 15, 1999; 5(5): 369-374
Published online Oct 15, 1999. doi: 10.3748/wjg.v5.i5.369
Published online Oct 15, 1999. doi: 10.3748/wjg.v5.i5.369
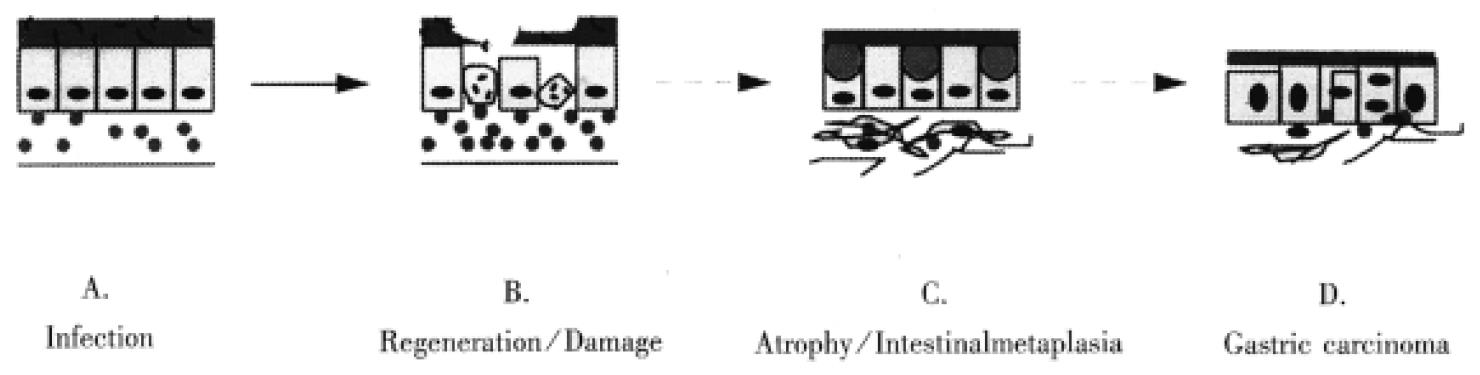
Figure 1 Long-term H.
pylori infection may induce a repeated “damage-regeneration” process which gradually leads to gastric atrophy, intestinal metaplasia and finally carcinoma. A. H. pylori infected gastric mucosa with inflammatory cell infiltration. B. Gastric epithelial cells are damaged, become apoptotic and then are regenerated, with enhanced inflammatory cell infiltration and disturbance of mucus layer. C. Atrophy and intestinal metaplasia develop; fibrosis and thinning of the lamina propria; finally H. pylori is lost due to inhospitable mucosa. D. Gastric carcinoma is induced.
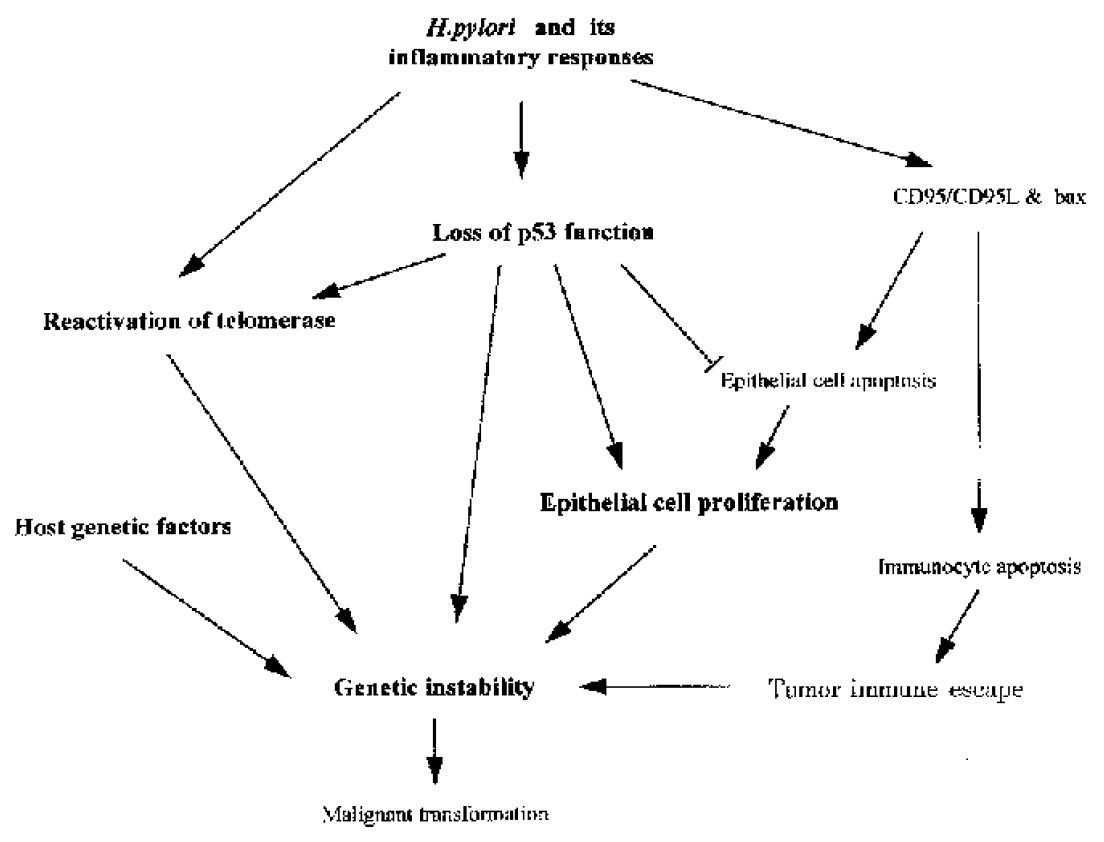
Figure 2 Possible molecular mechanisms of H.
pylori associated carcinogenesis. H. pylori and its associated inflammatory responses cause reactivation of telomerase, the loss of p53 function and also induce CD95 and bax mediated apoptosis. CD95L produced by gastric epithelial cells m ay also cause intra-mucosal immunocyte apoptosis, which could facilitate tumor immune escape. However, mutated p53 may attenuate epithelial cell apoptosis, providing a possible selective advantage for tumor cell proliferation. Furthermore , p53 deficiency may cooperate with telomere dysfunction to accelerate carcinogenesis. All of these changes, together with host genetic factors may play important roles in the development of H. pylori associated carcinogenesis.
- Citation: Zhang ZW, Farthing MJ. Molecular mechanisms of H. pylori associated gastric carcinogenesis. World J Gastroenterol 1999; 5(5): 369-374
- URL: https://www.wjgnet.com/1007-9327/full/v5/i5/369.htm
- DOI: https://dx.doi.org/10.3748/wjg.v5.i5.369