Copyright
©The Author(s) 2025.
World J Gastroenterol. Jul 21, 2025; 31(27): 109105
Published online Jul 21, 2025. doi: 10.3748/wjg.v31.i27.109105
Published online Jul 21, 2025. doi: 10.3748/wjg.v31.i27.109105
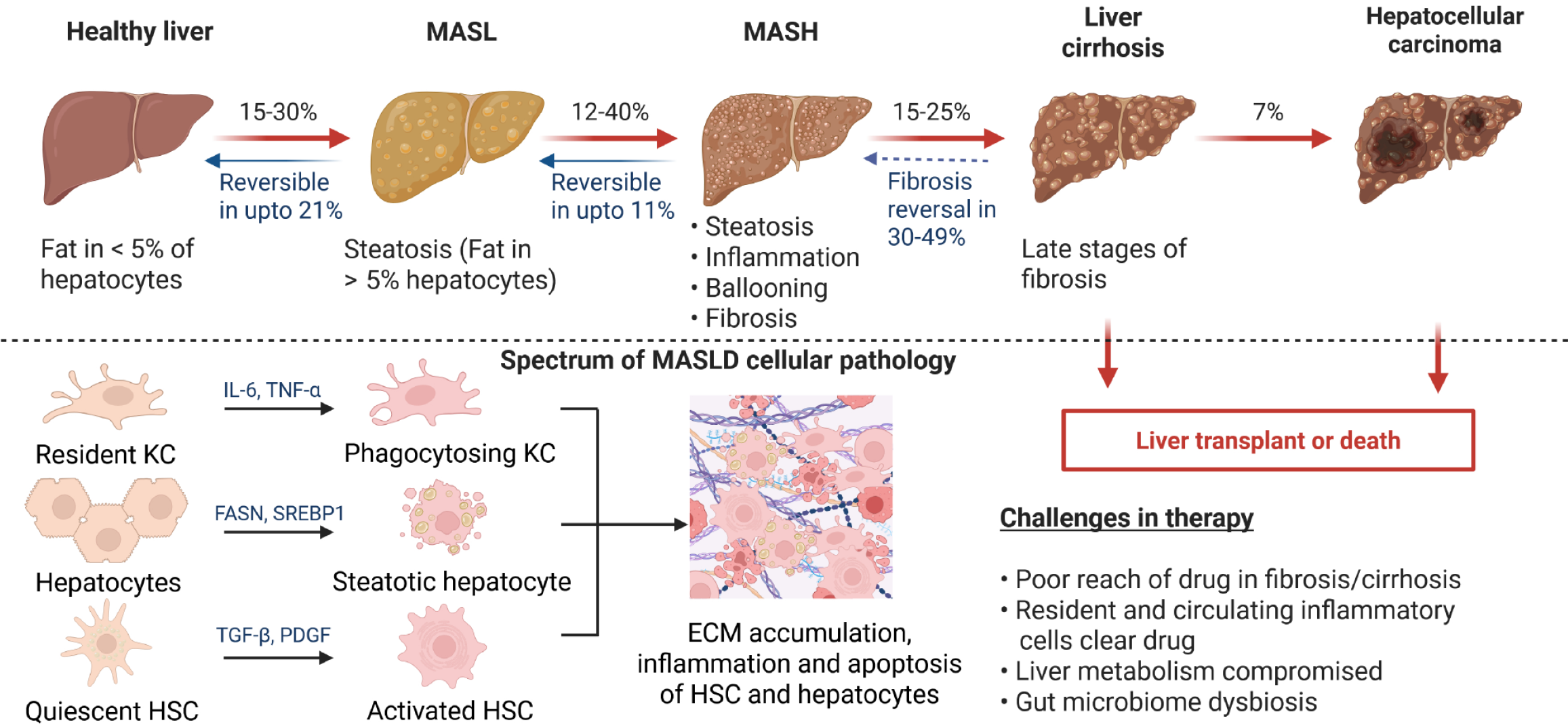
Figure 1 Spectrum of pathology of metabolic dysfunction-associated steatotic liver disease.
Regular intake of high fat loaded food can trigger the development of metabolic dysfunction-associated steatotic liver in 15%-30% of healthy population, which is characterized by steatosis, implying fat in > 5% hepatocytes and is reversible in up to 21% of patients. In 12%-40% of patients metabolic dysfunction-associated steatotic liver may progress towards the more aggressive form of the disease metabolic dysfunction-associated steatohepatitis (MASH), which is characterized by steatosis, inflammation, ballooning of hepatocytes and fibrosis. MASH is reversible in up to 11% patients. Among 15%-25% MASH patients there is high risk of the development of liver cirrhosis characterized by severely compromised liver function and 7% of cirrhotic patients may develop liver cancer. The only therapeutic option for liver cirrhosis and liver cancer is liver transplantation. At the cellular level, hepatocytes develop steatosis under the influence of enzymes such as fatty acid synthase, and sterol regulatory element binding protein 1. Persistent steatosis induces hepatic inflammation involving immune cells including Kupffer cells. Long-standing inflammation will activate quiescent hepatic stellate cells to cause fibrosis up to cirrhosis by excessive synthesis of extracellular matrix proteins. Cirrhosis may give rise to hepatocellular carcinoma. The interplay of different hepatic cells culminates in the pathological features of MASH. MASL: Metabolic dysfunction-associated steatotic liver; MASH: Metabolic dysfunction-associated steatohepatitis; MASLD: Metabolic dysfunction-associated steatotic liver disease; ECM: Extracellular matrix; HSC: Hepatic stellate cell; KC: Kupffer cells; IL-6: Interleukin-6; TNF-α: Tumor necrosis factor-alpha; FASN: Fatty acid synthase; SREBP1: Sterol regulatory element binding protein 1; TGF-β: Transforming growth factor-beta; PDGF: Platelet-derived growth factor. Created with a license from BioRender.com.
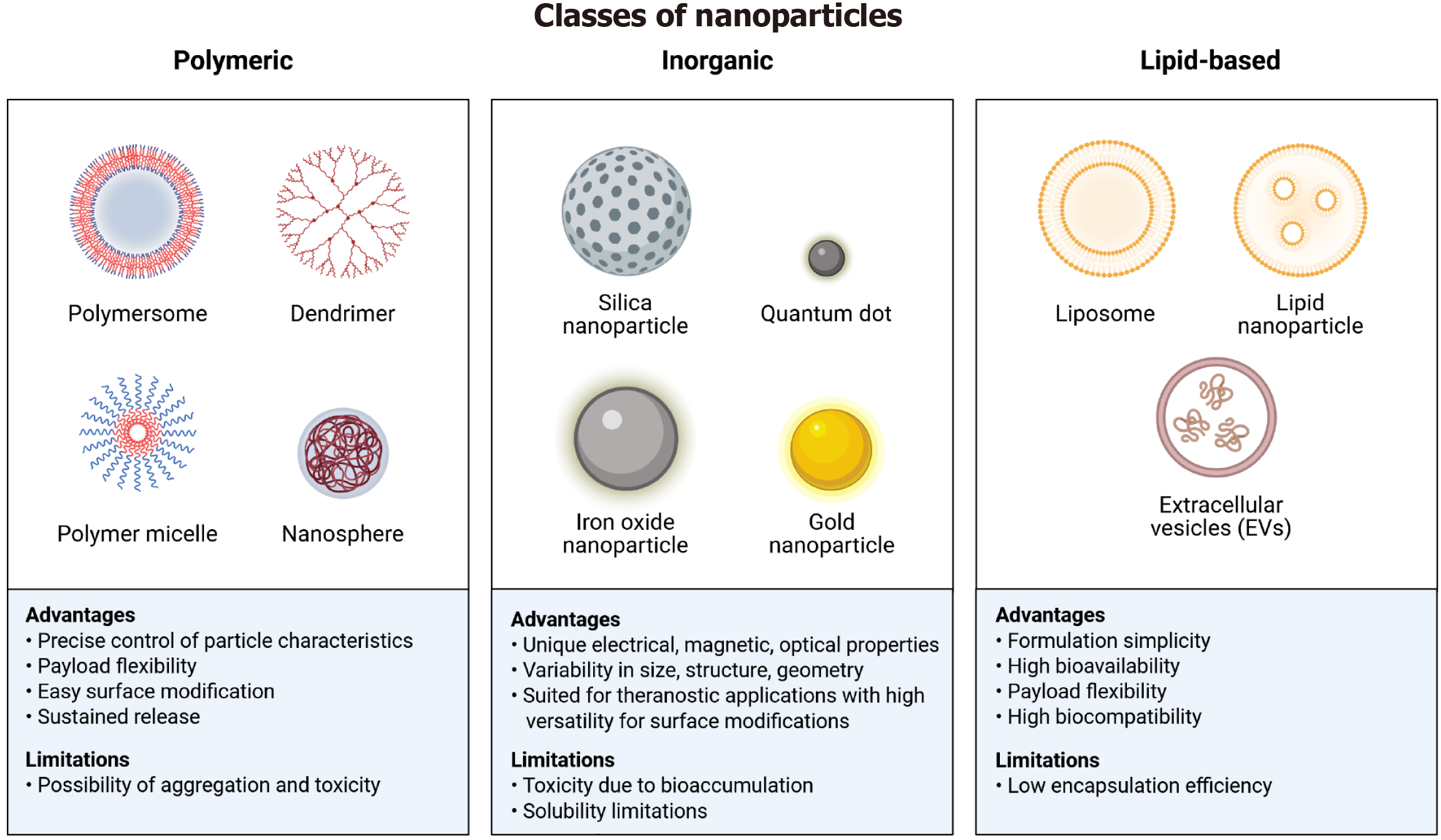
Figure 2 Classification of different types of nanoparticles with their specific advantages and disadvantages.
The figures enlist three major types of nanoparticles: Polymeric, inorganic, and lipid-based. The polymeric nanoparticles include polymersomes, dendrimers, polymer micelles, and nanospheres. Advantages of polymeric nanoparticles are precise control, flexibility, surface modification, and sustained release; while the limitations include risk of toxicity and aggregation. The inorganic nanoparticles comprise silica nanoparticles, quantum dots, iron oxide nanoparticles, and gold nanoparticles. Their advantages are the unique physicochemical properties, tailored design, and versatility. Their solubility and toxicity might pose challenges in delivery. The lipid-nanoparticles can be subdivided into liposomes, lipid nanoparticles, and extracellular vesicles. They offer advantages such as simplicity, high bioavailability, payload flexibility, and high biocompatibility, whereas the low encapsulation remains a limitation. Created with a license from BioRender.com.
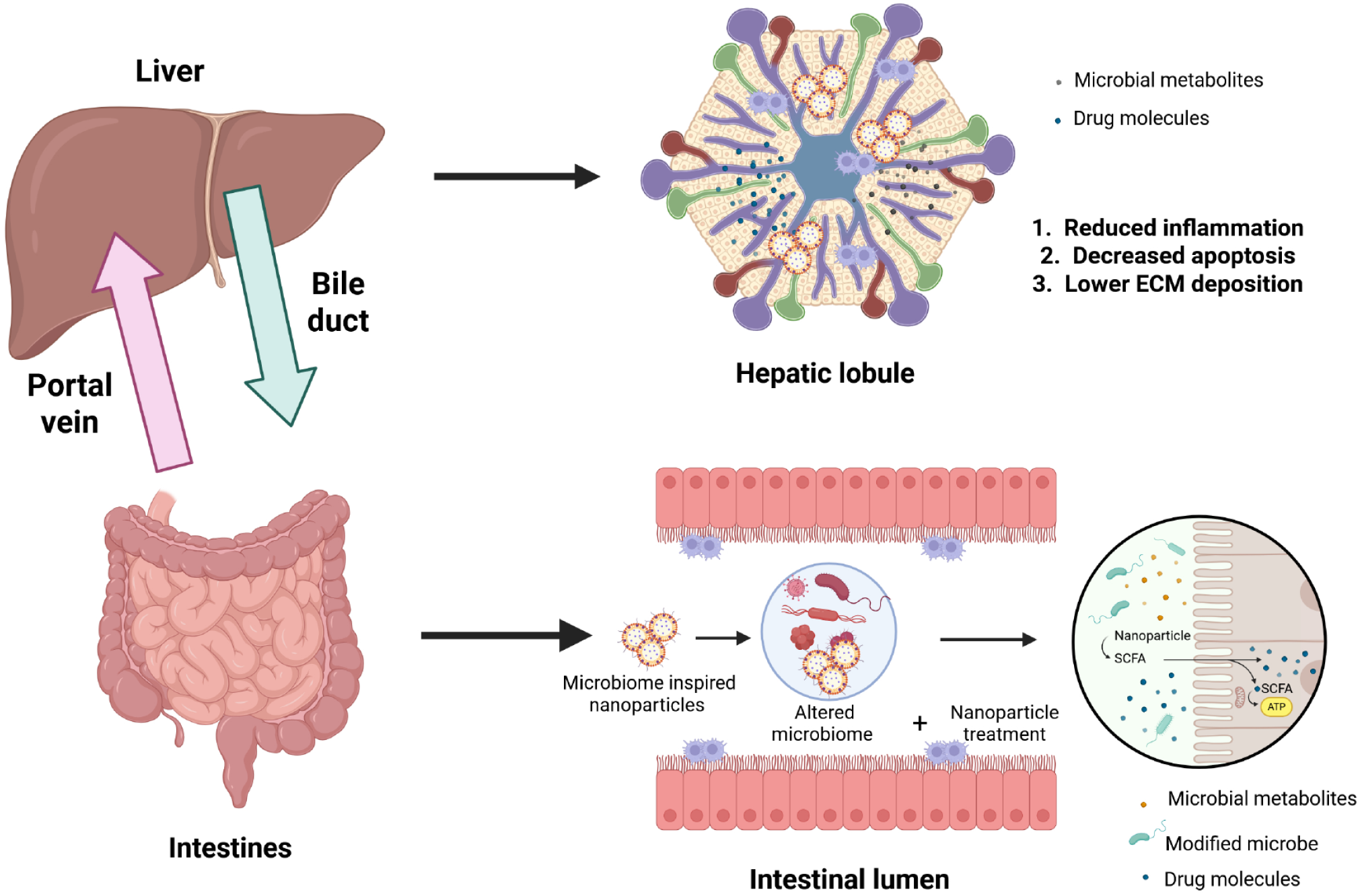
Figure 3 Mechanism of action of orally delivered microbiome nanocarriers for the treatment of liver diseases.
The enterohepatic circulation plays an important role in the mobility of nanoparticles, wherein the portal vein circulates nanoparticles and their payload to the liver, and the metabolites may then circle back again from the liver to the intestine via the biliary system. In the intestine, microbiome-inspired nanoparticles may potentially affect the microbiome by modifying the microbiome profile, by delivering pharmacologically active microbial metabolites (such as short chain fatty acids) or molecules. Within the liver, liposomes may reduce inflammation by acting on immune cells such as Kupffer cells, may reduce apoptosis of hepatocytes, and/or may reduce the deposition of extracellular matrix proteins including collagen, elastin, and fibronectin. ECM: Extracellular matrix; SCFA: Short chain fatty acids. Created with a license from BioRender.com.
- Citation: Khurana A, Hartmann P. Gut microbiome-specific nanoparticle-based therapeutics for liver diseases. World J Gastroenterol 2025; 31(27): 109105
- URL: https://www.wjgnet.com/1007-9327/full/v31/i27/109105.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i27.109105