Copyright
©The Author(s) 2025.
World J Gastroenterol. Jun 7, 2025; 31(21): 106939
Published online Jun 7, 2025. doi: 10.3748/wjg.v31.i21.106939
Published online Jun 7, 2025. doi: 10.3748/wjg.v31.i21.106939
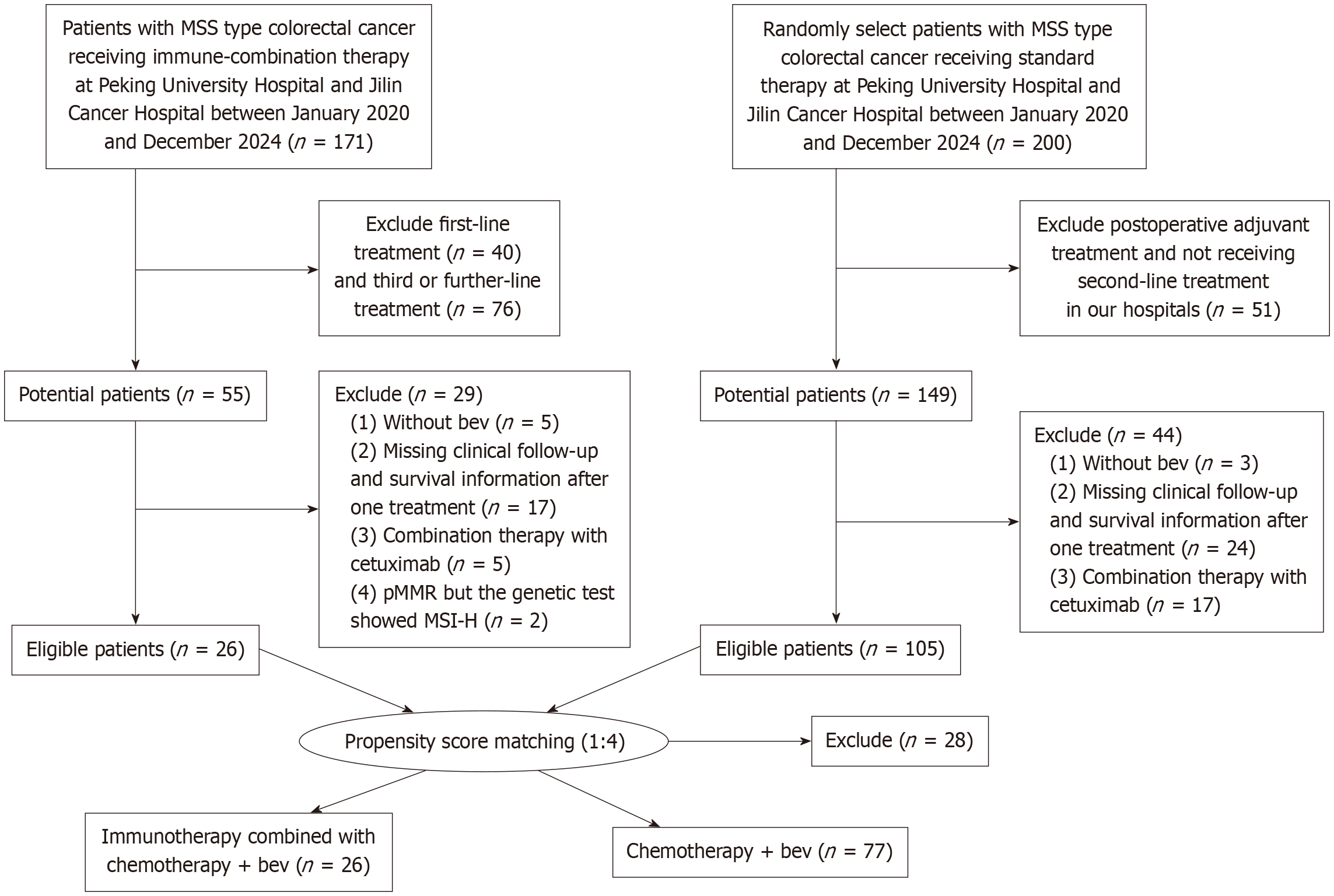
Figure 1 Flow chart of patient inclusion.
MSS: Microsatellite stable; bev: Bevacizumab; pMMR: Proficient mismatch repair; MSI-H: Microsatellite instability-high.
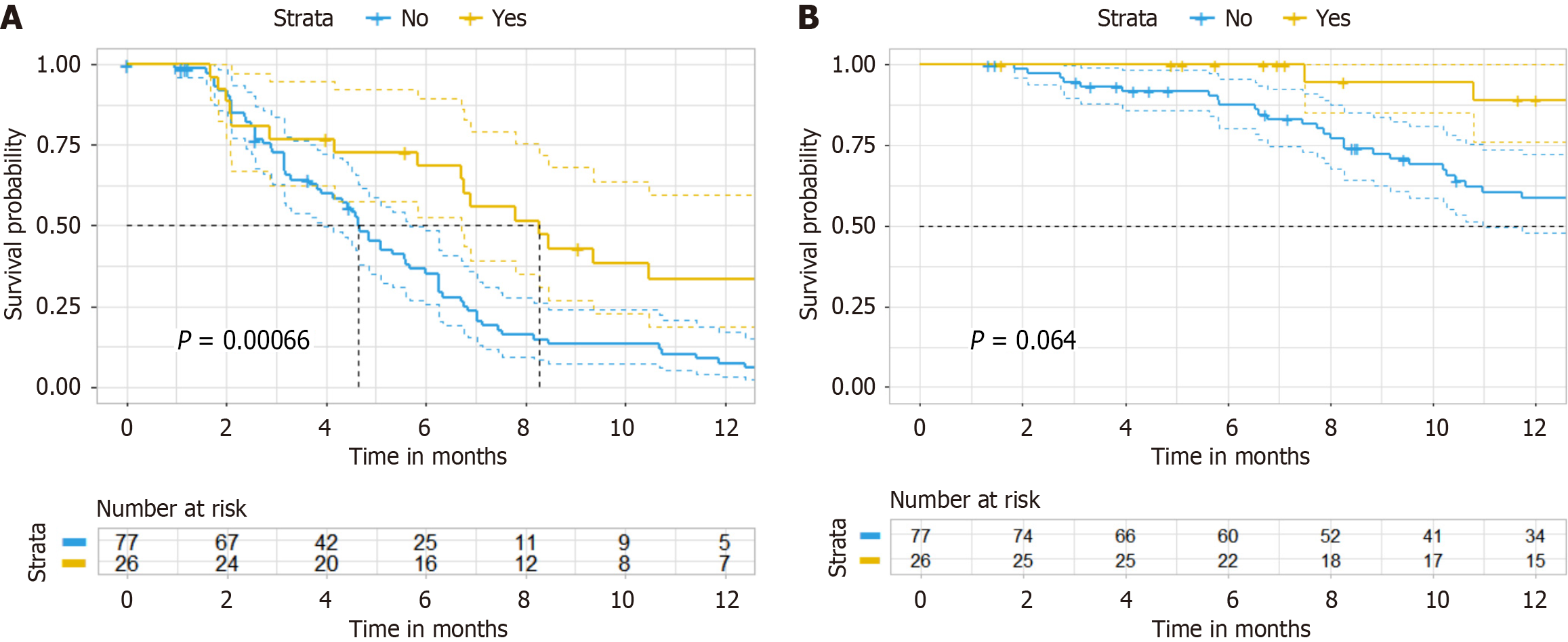
Figure 2 After propensity score-matching analysis of progression-free survival and overall survival.
A: After propensity score-matching analysis of PFS (ratio = 4); B: After propensity score-matching analysis of overall survival (ratio = 4).
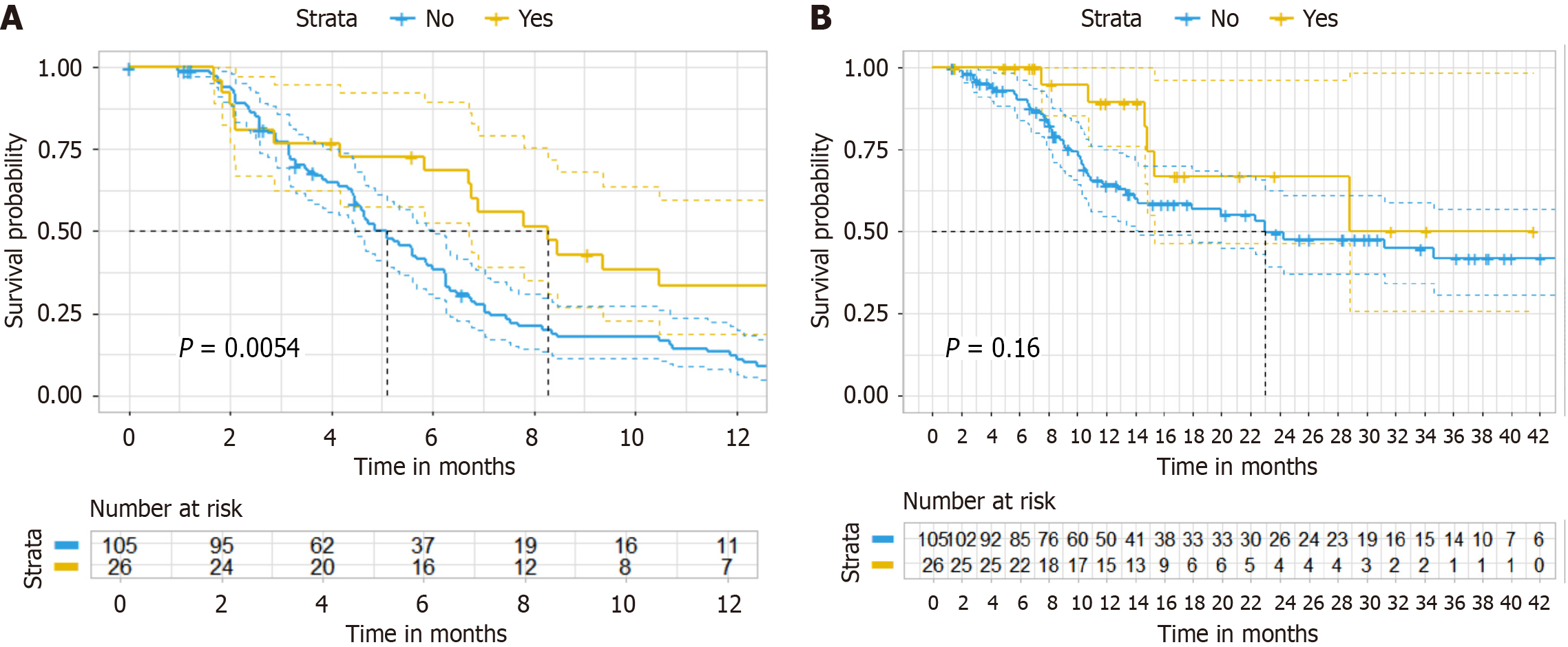
Figure 3 Kaplan-Meier curve of original cohort.
A: In the original cohort (progression-free survival); B: In the original cohort (overall survival).
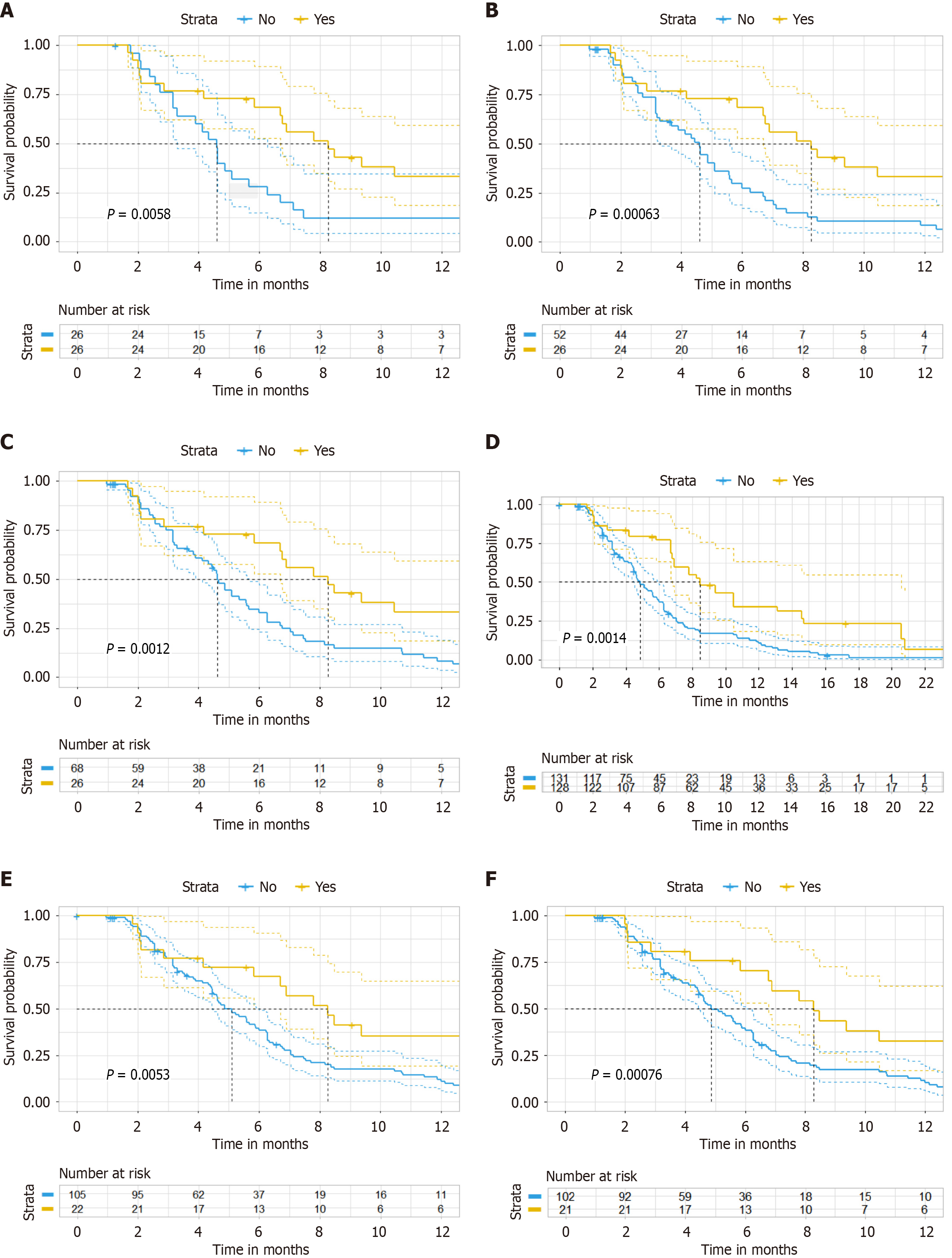
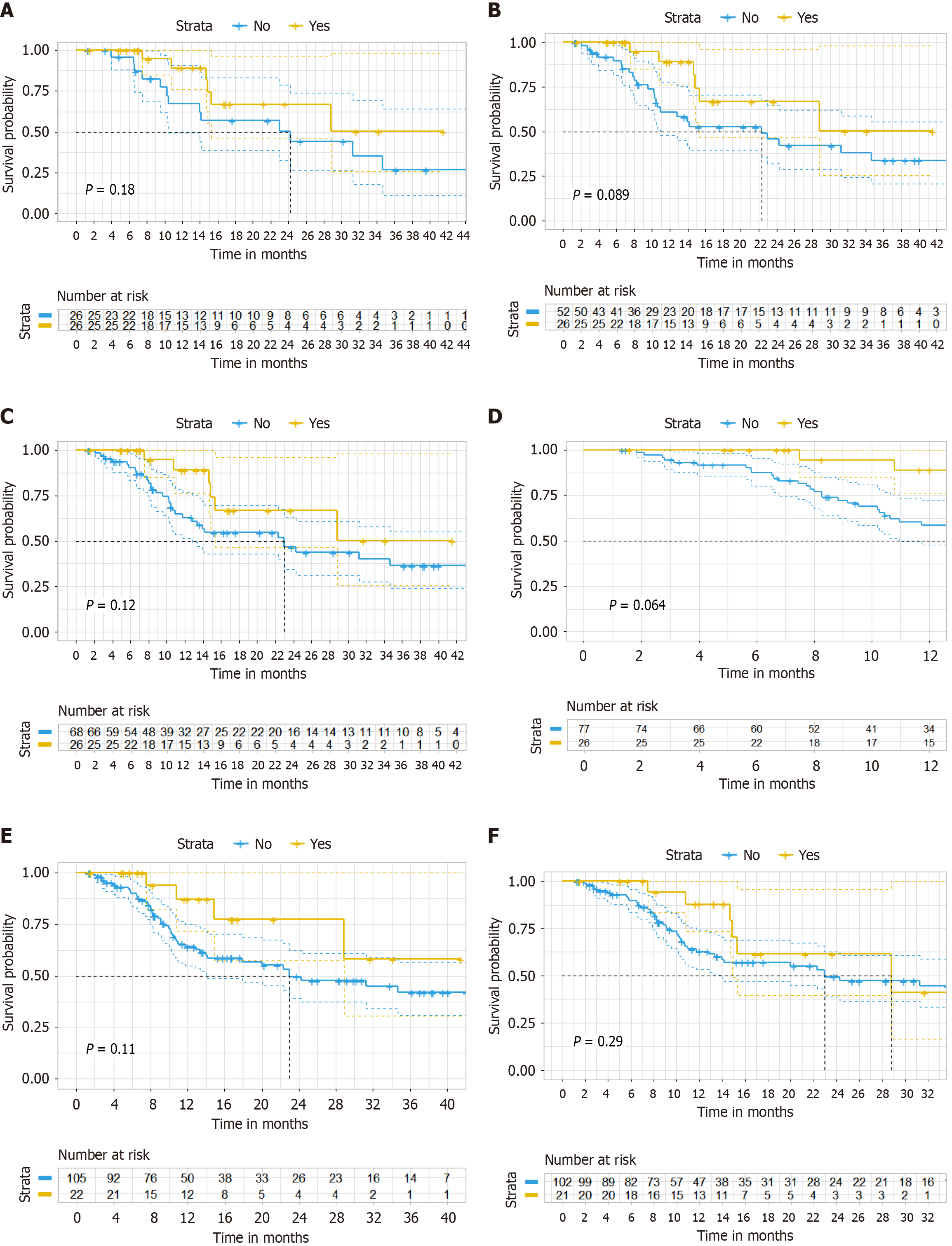
Figure 4 Sensitivity analysis of progression-free survival.
A: After propensity score-matching analysis in progression-free survival (PFS) (ratio = 1); B: After propensity score-matching analysis in PFS (ratio = 2); C: After propensity score-matching analysis in PFS (ratio = 3); D: After inverse probability of treatment weighting analysis; E: Kaplan-Meier curve after excluding 4 patients who received single-agent chemotherapy combined with bevacizumab and anti- programmed death 1 immunotherapy in PFS; F: Kaplan-Meier curve restricting the cohort to irinotecan-based chemotherapy patients in PFS.
Figure 5 Sensitivity analysis of overall survival.
A: After propensity score-matching analysis in overall survival (OS) (ratio = 1); B: After propensity score-matching analysis in OS (ratio = 2); C: After propensity score-matching analysis in OS (ratio = 3); D: After inverse probability of treatment weighting analysis (OS); E: Kaplan-Meier curve after excluding 4 patients who received single-agent chemotherapy combined with bevacizumab and anti-programmed death 1 immunotherapy in OS; F: Kaplan-Meier curve restricting the cohort to irinotecan-based chemotherapy patients in OS.
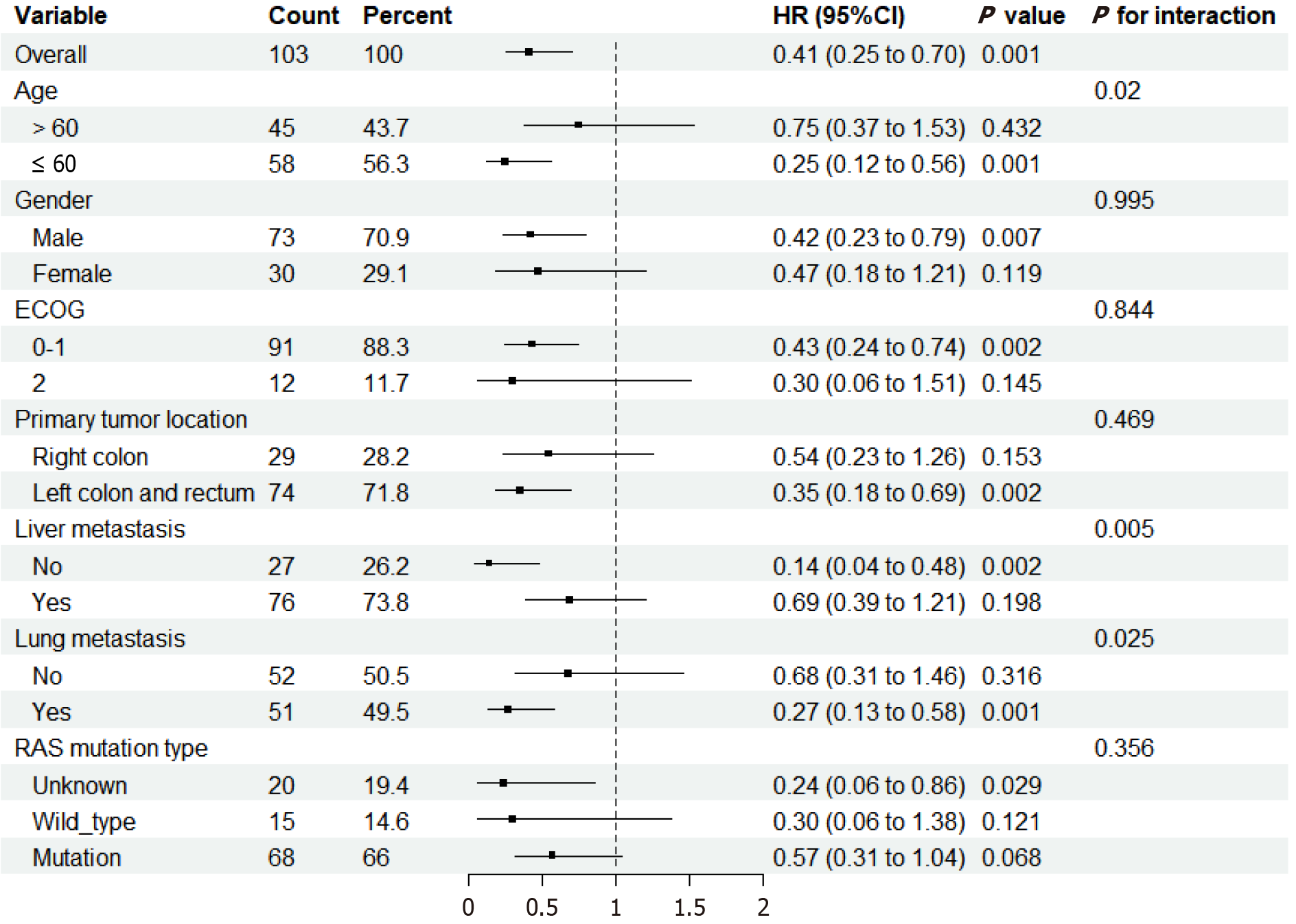
Figure 6 Forest plots depict the hazard ratios and 95% confidence intervals for progression-free survival by subgroup.
CI: Confidence interval; ECOG: Eastern Cooperative Oncology Group.
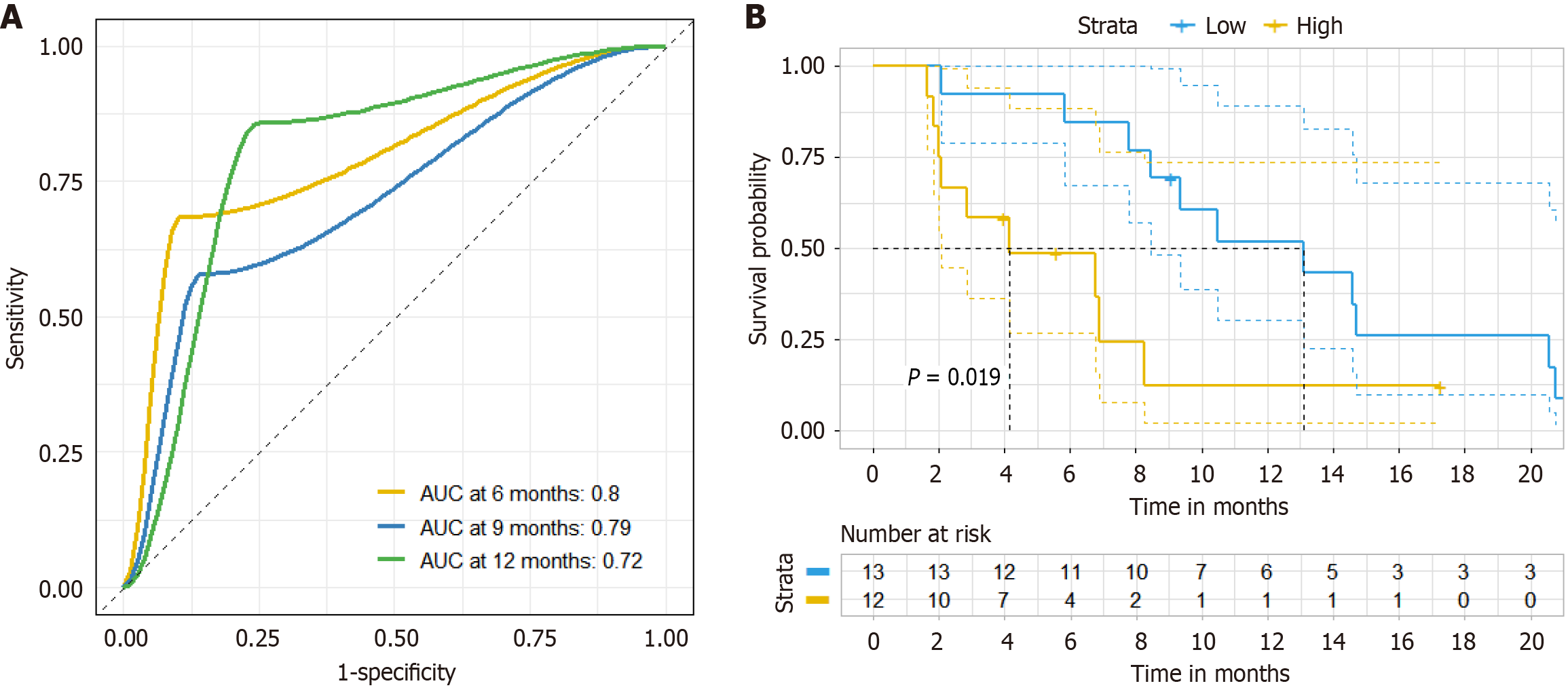
Figure 7 Lactate dehydrogenase as a predictive biomarker of progression-free survival.
A: Receiver operating characteristic curves of lactate dehydrogenase (LDH) at 9 months, 12 months, and 15 months before patients receiving chemotherapy combined with bevacizumab and anti-programmed death 1 immunotherapy; B: Survival curves of LDH before patients receiving chemotherapy combined with bevacizumab and anti-programmed death 1 immunotherapy (high group: LDH ≥ 240 mmol/L and low group LDH < 240 mmol/L). ACU: Area under curve.
- Citation: Gao Z, Wang XY, Shen ZG, Liu JH, Wang XY, Wu SK, Jin X. Chemotherapy plus bevacizumab with or without anti-programmed death 1 immunotherapy as the second-line therapy in colorectal cancer. World J Gastroenterol 2025; 31(21): 106939
- URL: https://www.wjgnet.com/1007-9327/full/v31/i21/106939.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i21.106939