Copyright
©The Author(s) 2025.
World J Gastroenterol. May 14, 2025; 31(18): 105729
Published online May 14, 2025. doi: 10.3748/wjg.v31.i18.105729
Published online May 14, 2025. doi: 10.3748/wjg.v31.i18.105729
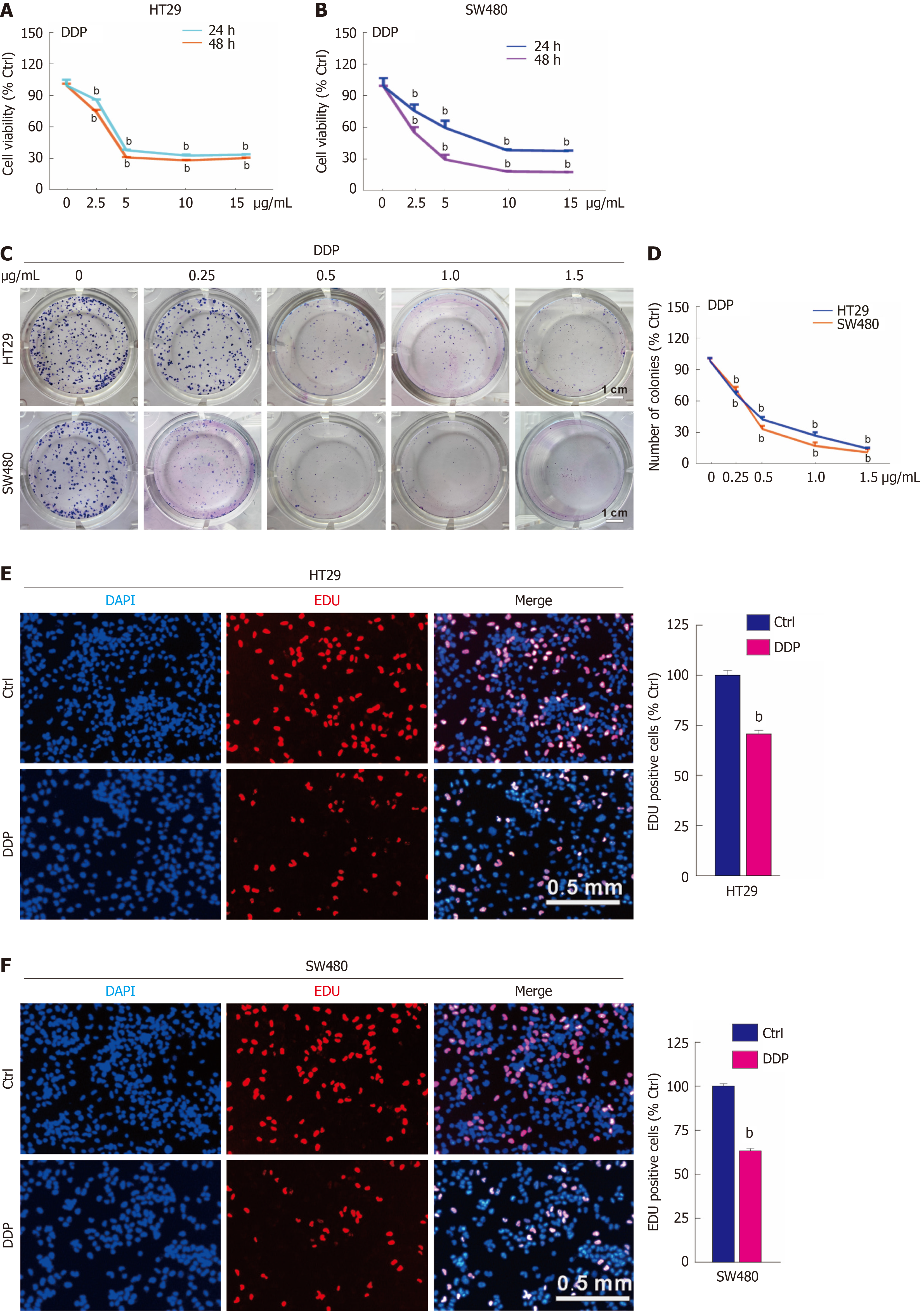
Figure 1 Cisplatin reduces cell proliferation in colorectal cancer.
A and B: Indicated concentrations of cisplatin (DDP) (μg/mL) were used to treat HT29 and SW480 cells, and the cell viability was measured by MTS assay; C and D: For the colony growth assay, HT29 and SW480 were treated with the indicated concentration gradient of DDP (μg/mL), and colony formation numbers were calculated and graphically represented using ImageJ and Prism GraphPad software. Scale bar = 1 cm; E and F: EdU staining assay was conducted to evaluate cell proliferation following DDP treatment (5 μg/mL, hereafter unless otherwise indicated) for 3 hours. Scale bar = 0.5 mm. bP < 0.01 vs control (Ctrl).
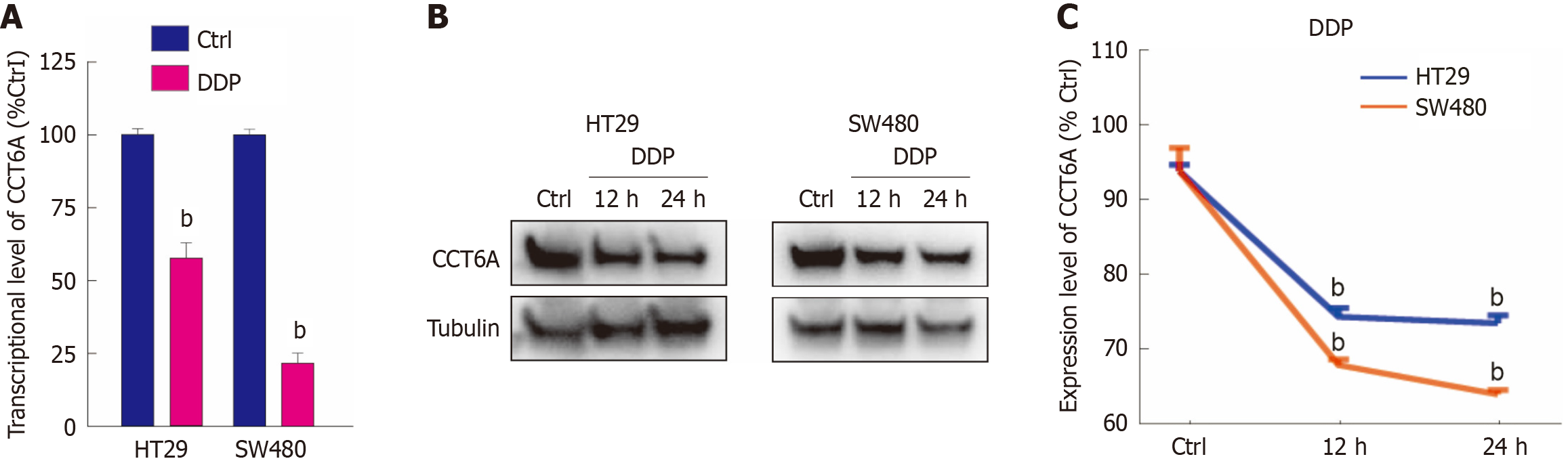
Figure 2 Cisplatin decreases the protein level of chaperonin-containing tailless complex polypeptide 1 subunit 6a.
A: Quantitative PCR was conducted to detect the transcription level of chaperonin-containing tailless complex polypeptide 1 subunit 6a (CCT6A) in both HT29 and SW480 cells following cisplatin (DDP) treatment for 4 hours; B and C: Following treatment with DDP for the indicated period, the whole protein was extracted and subjected to immunoblot analysis with the indicated antibodies. bP < 0.01 vs control (Ctrl).
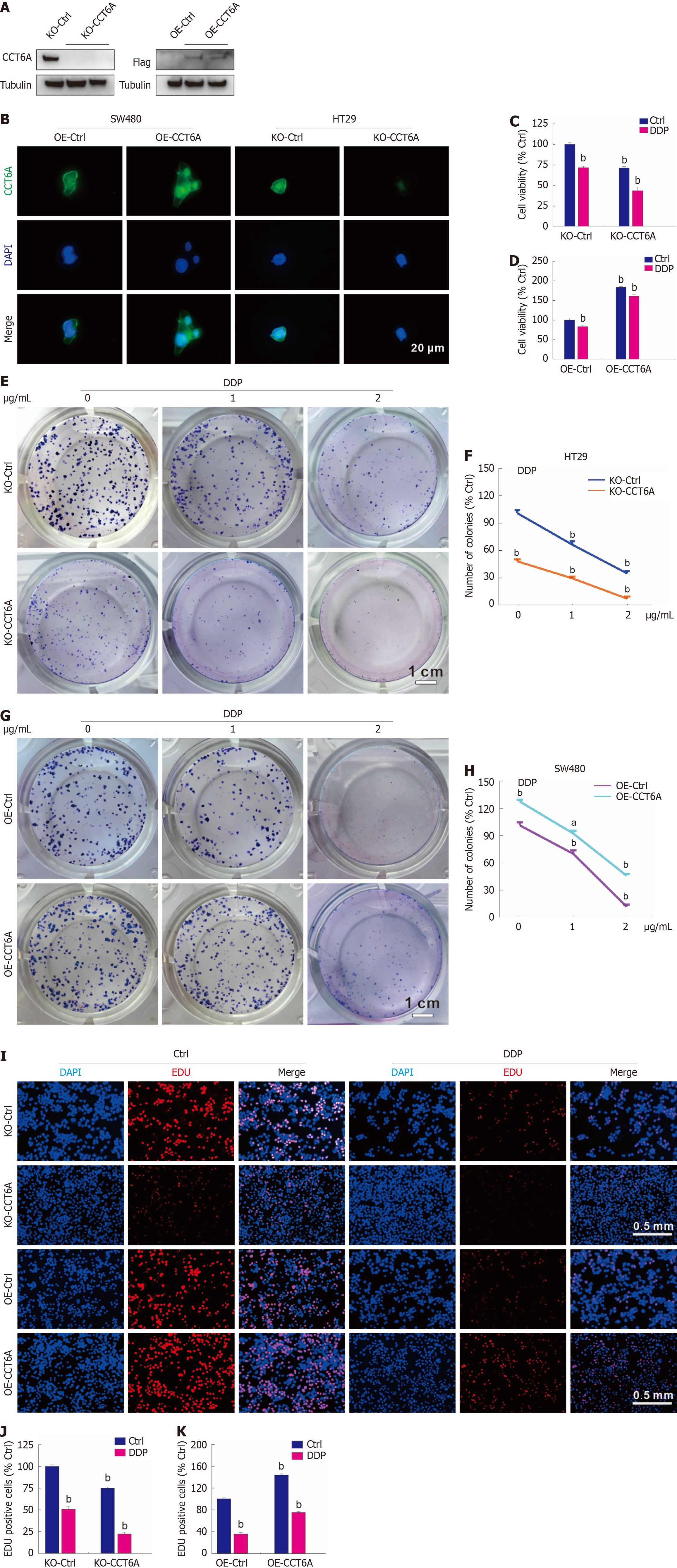
Figure 3 Chaperonin-containing tailless complex polypeptide 1 subunit 6a rescues the cell viability loss induced by cisplatin.
A and B: The knockout (KO)-chaperonin-containing tailless complex polypeptide 1 subunit 6A (CCT6A) HT29 cells and overexpression (OE)-CCT6A SW480 cells were constructed as described in Methods, and the efficacies were confirmed by immunoblotting and immunofluorescence, respectively. Scale bar = 20 μm; C and D: Utilizing the MTS assay, the cell viability was identified in the indicated cells in the presence or absence of cisplatin (DDP); E-H: Cell proliferation in the indicated cells with or without DDP (μg/mL) treatment was analyzed by colony growth assay (scale bar = 1 cm); I-K: EdU staining assay was performed in the indicated cells with or without DDP treatment (scale bar = 0.5 mm). aP < 0.05 vs control (Ctrl); bP < 0.01 vs Ctrl.
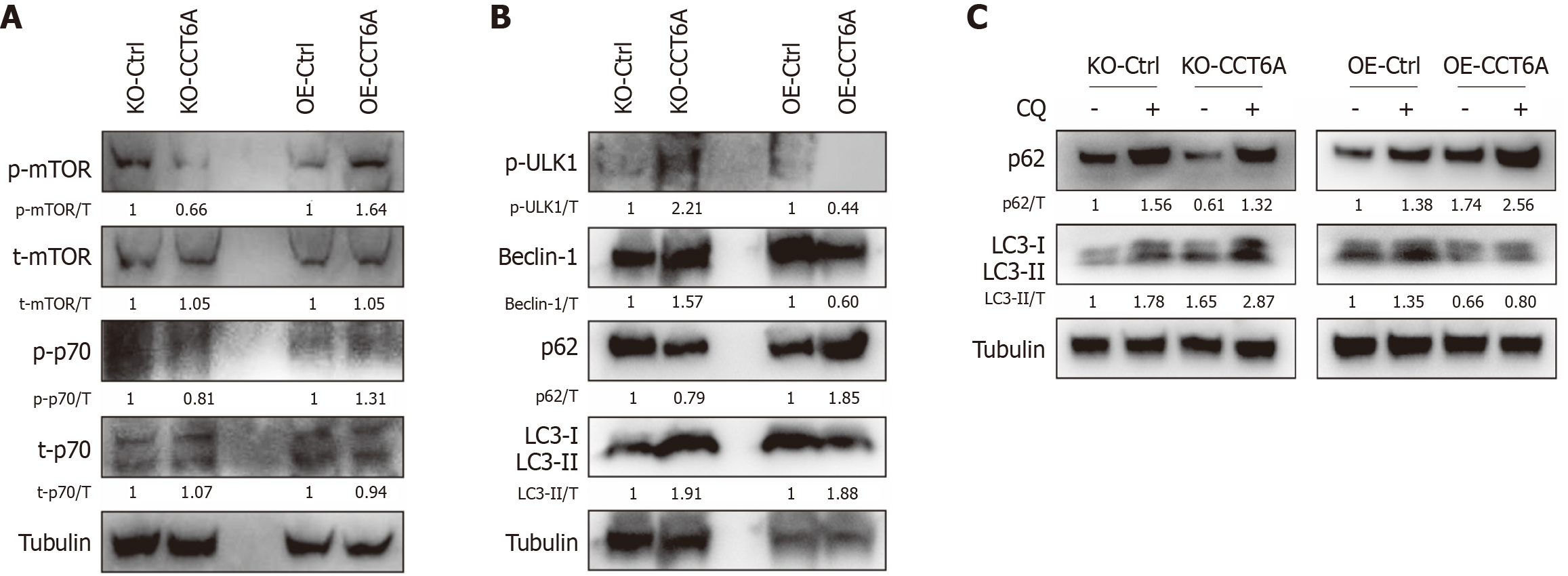
Figure 4 Chaperonin-containing tailless complex polypeptide 1 subunit 6A affects the autophagy pathway in colorectal cancer cells.
A and B: Cell lysates were extracted from knockout (KO)-Control (Ctrl) and KO-chaperonin-containing tailless complex polypeptide 1 subunit 6A (CCT6A) HT29 cells, as well as overexpression (OE)-Ctrl and OE-CCT6A SW480 cells, and then subjected to immunoblotting using the indicated antibodies; C: Following treatment with chloroquine diphosphate salt (CQ, 20 μM, hereafter unless otherwise indicated) for 2 hours, immunoblotting was performed with the total proteins extracted from the indicated cells. DDP: Cisplatin; LC3: Light chain 3; mTOR: Mammalian target of rapamycin; Ulk1: Unc-51-like autophagy activating kinase 1.
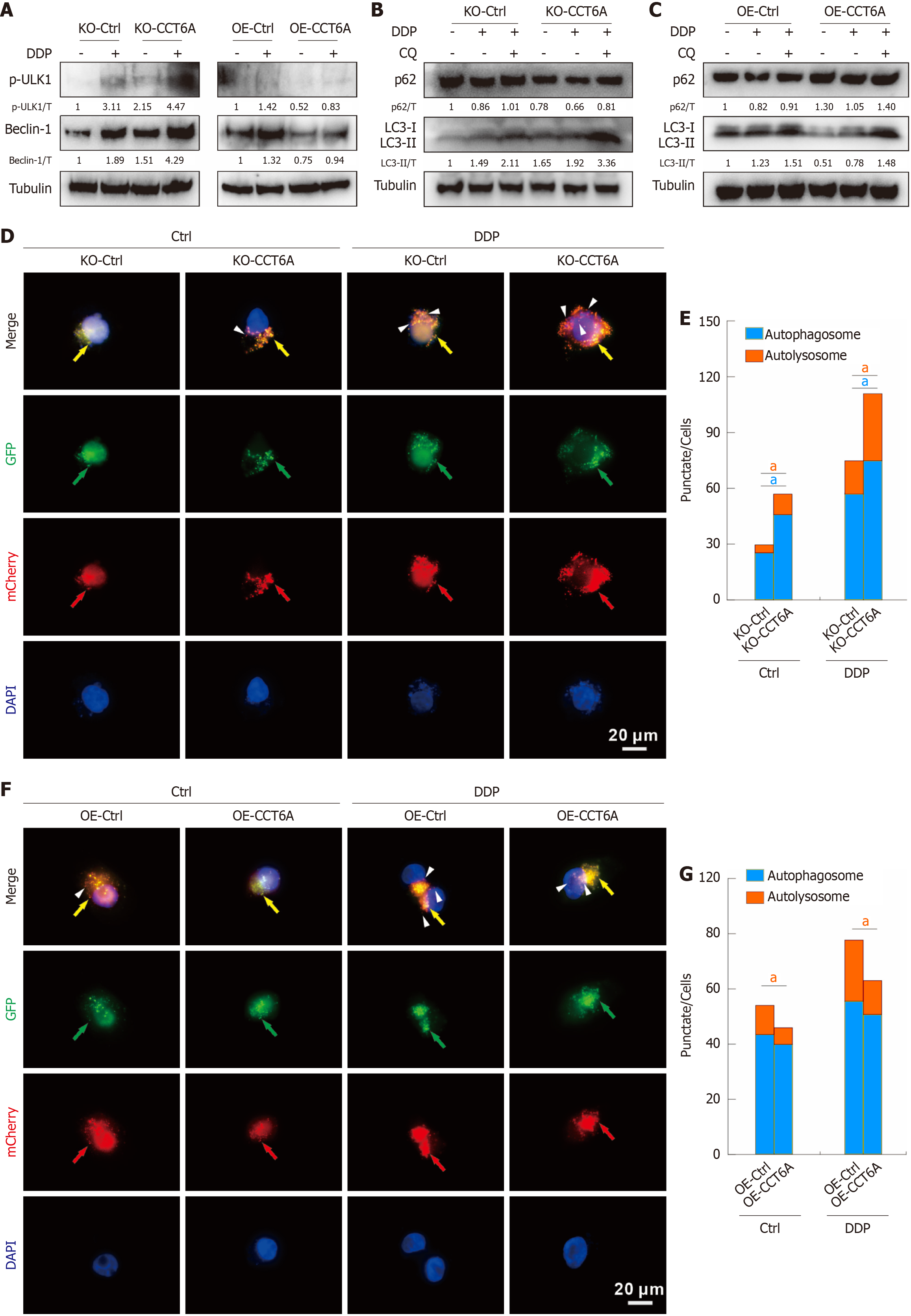
Figure 5 Chaperonin-containing tailless complex polypeptide 1 subunit 6A negative modulates autophagy induced by cisplatin in colorectal cancer.
A-C: Cells were treated with or without cisplatin (DDP) in the presence or absence of CQ for 2 hours, cell lysates were extracted and performed immunoblotting with indicated antibodies; D-G: Following transfection with mCherry-GFP-LC3B adenovirus for 24 hours, cells were split onto coverslips, and then captured by immunofluorescence microscopy (1000 magnification; green arrow: green fluorescence protein (GFP) dot; red arrow: MCherry dot; yellow arrow: Merge dot; white arrowhead: Sole red dot; scale bar = 20 μm). aP < 0.05 vs control (Ctrl); CCT6A: Chaperonin-containing tailless complex polypeptide 1 subunit 6a; CQ: Chloroquine diphosphate salt; KO: Knockout; OE: Overexpression.
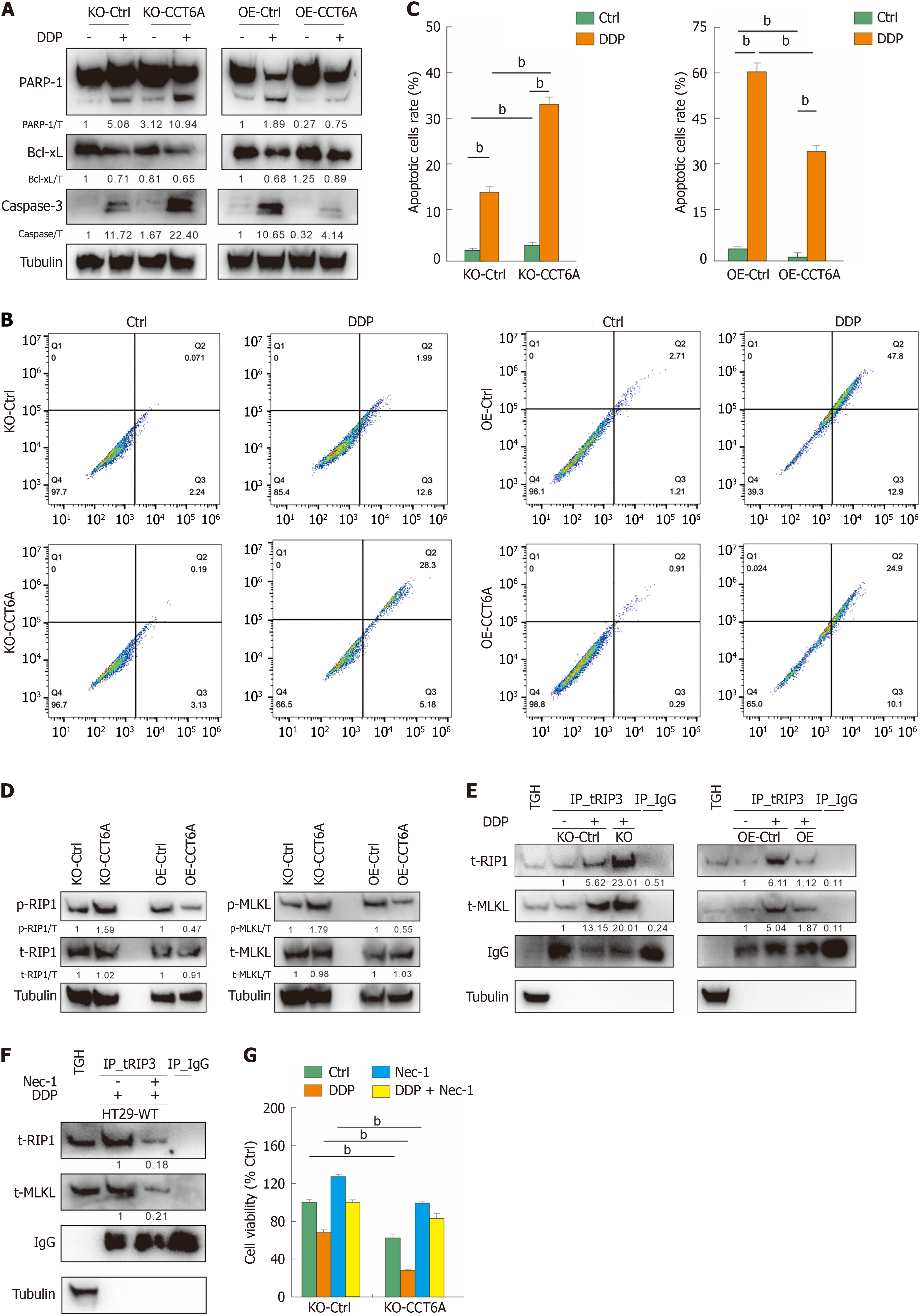
Figure 6 Chaperonin-containing tailless complex polypeptide 1 subunit 6A negatively modulates apoptosis and necroptosis in colorectal cancer.
A: After treating the indicated cells with cisplatin (DDP) for 24 hours, cell lysates were prepared and subjected to immunoblotting; B and C: Following treatment of the cells with DDP for 24 hours, the apoptosis were determined by flow cytometry (Annexin V-positive), and the data were visualized in histograms; D: Following treatment with DDP for 24 hours, cell lysates were performed immunoblotting with indicated antibodies; E and F: Cells were exposed to the appropriate drugs for 24 hours (necrostatin-1 [Nec-1]: 10 μM, hereafter unless otherwise indicated), cells were lysed, and immunoprecipitated using the receptor-interacting protein kinase 3 (RIP3) antibody. The immunoprecipitates were resolved by electrophoresis and probed by immunoblotting with the indicated antibodies; G: Cell viability in knockout (KO)-Control (Ctrl) and KO-chaperonin-containing tailless complex polypeptide 1 subunit 6a (CCT6A) cells was analyzed following the indicated treatments for 24 hours. bP < 0.01 vs control. Bcl-xL: B-cell lymphoma-extra large; IgG: Immunoglobulin G (negative control antibody); MLKL: Mixed lineage kinase domain-like; Nec-1; Necrostatin-1; OE: Overexpression; PARP-1; Poly(ADP-ribose) polymerase 1; TH: Total homogenate.
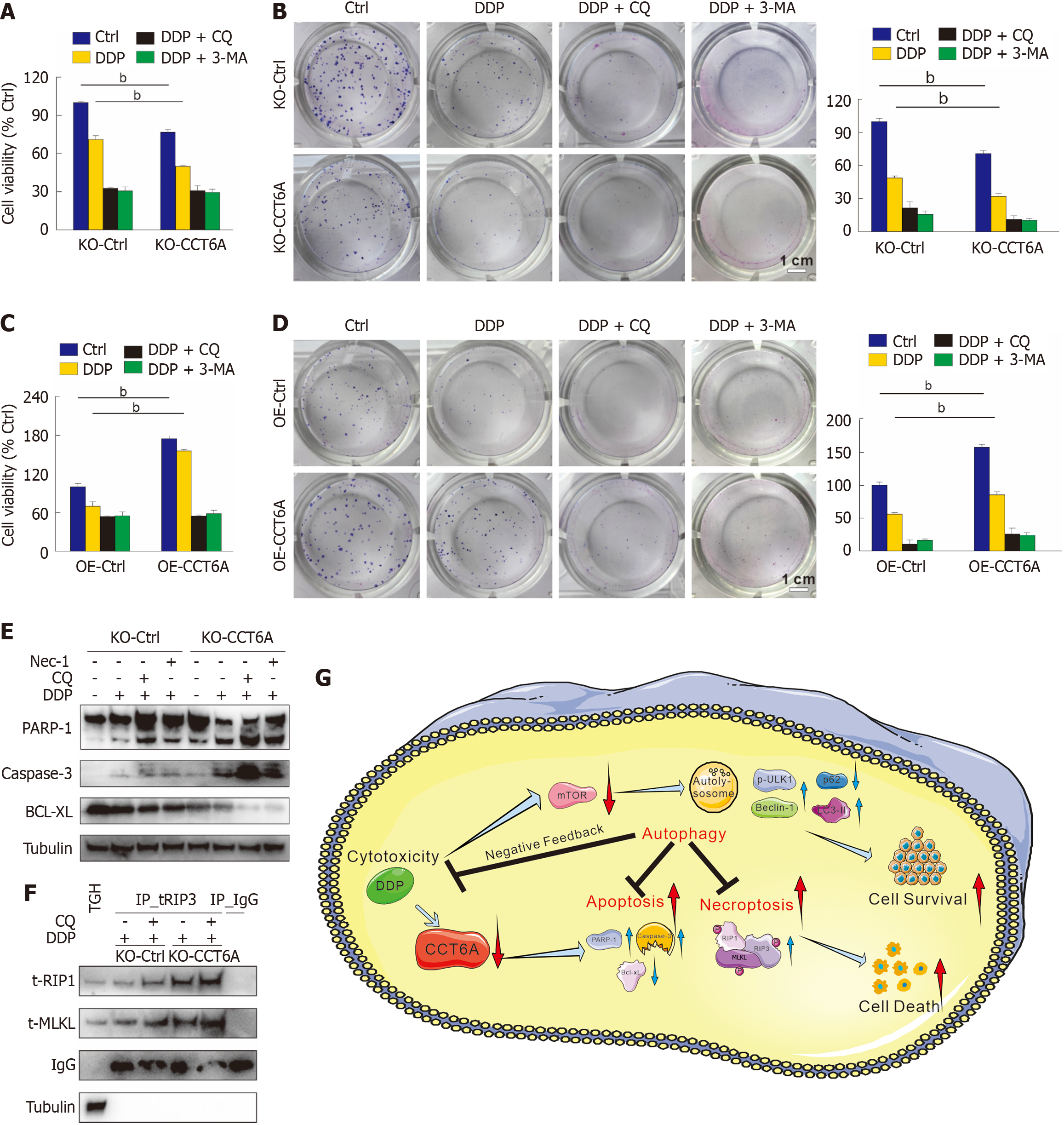
Figure 7 Inhibition of autophagy enhances the cytotoxicity of cisplatin.
A-D: The MTS assay was conducted to assess the cell viability after applying the specified treatments for 24 hours (3-Methyladenine [3-MA]: 2 mM) (A and C), colony growth assay was performed with indicated treatments (cisplatin [DDP]: 1 μM; chloroquine diphosphate salt [CQ]: 5 μM; 3-MA: 0.5 mmol/L) (scale bar = 1 cm) (B and D); E: Following appropriate treatments for 24 hours, cell lysates were performed immunoblotting with indicated antibodies; F: Co-immunoblotting assay was conducted with the receptor-interacting protein kinase 3 antibody, and then subjected to immunoblotting; G: Schematic model of chaperonin-containing tailless complex polypeptide 1 subunit 6a (CCT6A) modulates the cytotoxicity of DDP via regulating autophagy. bP < 0.01 vs control (Ctrl). Bcl-xL: B-cell lymphoma-extra large; IgG: Immunoglobulin G (negative control antibody); MLKL: Mixed lineage kinase domain-like; Nec-1; Necrostatin-1; PARP-1; Poly(ADP-ribose) polymerase 1; RIP1: Receptor-interacting protein kinase 1; TH: Total homogenate.
- Citation: Ma JX, Li XJ, Li YL, Liu MC, Du RH, Cheng Y, Li LJ, Ai ZY, Jiang JT, Yan SY. Chaperonin-containing tailless complex polypeptide 1 subunit 6A negatively regulates autophagy and protects colorectal cancer cells from cisplatin-induced cytotoxicity. World J Gastroenterol 2025; 31(18): 105729
- URL: https://www.wjgnet.com/1007-9327/full/v31/i18/105729.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i18.105729