Copyright
©The Author(s) 2025.
World J Gastroenterol. Apr 21, 2025; 31(15): 104901
Published online Apr 21, 2025. doi: 10.3748/wjg.v31.i15.104901
Published online Apr 21, 2025. doi: 10.3748/wjg.v31.i15.104901
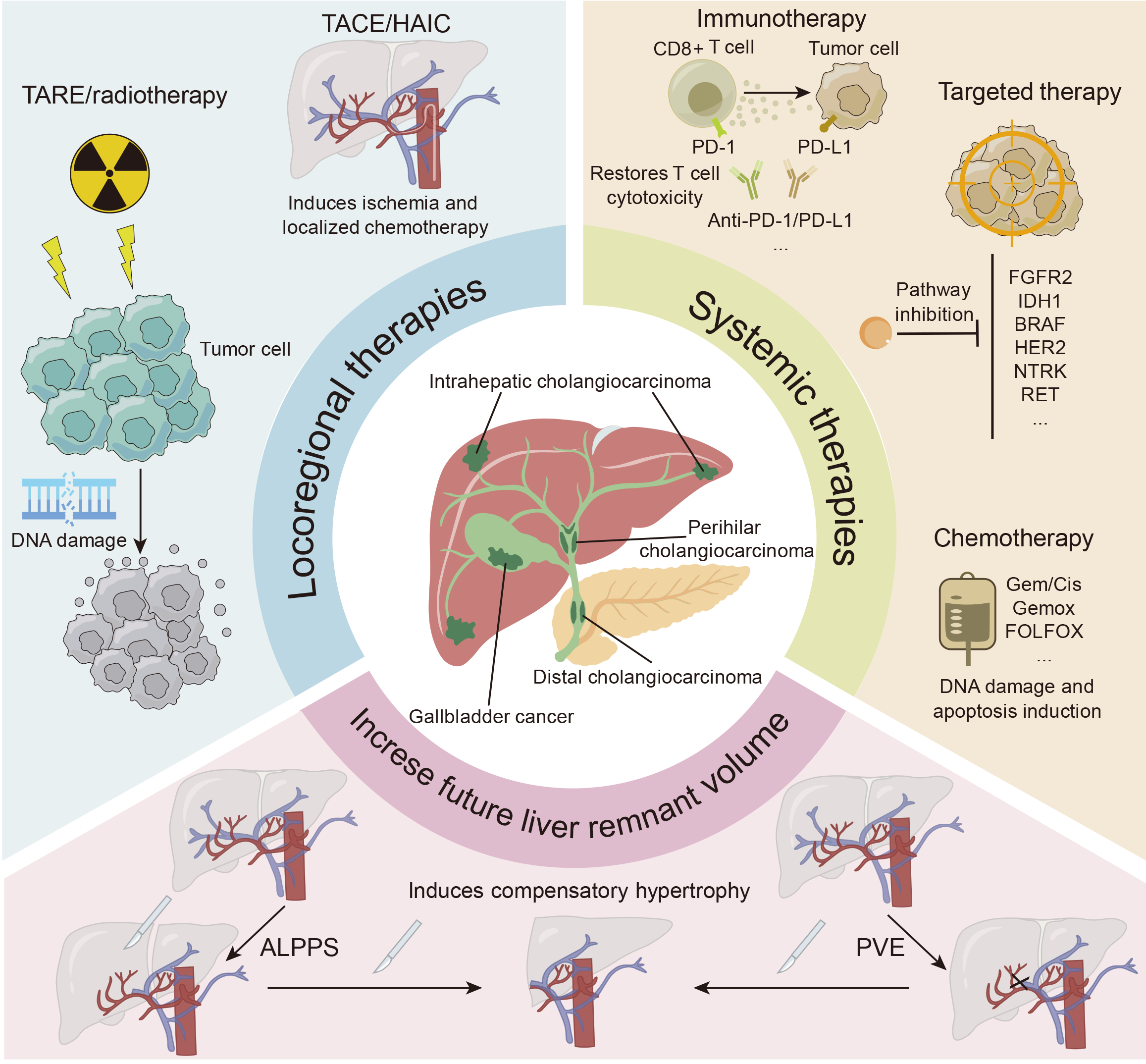
Figure 1 Schematic presentation of multiple conversion treatment options for intrahepatic cholangiocarcinoma.
TACE: Transarterial chemoembolization; HAIC: Hepatic arterial infusion chemotherapy; TARE: Transarterial radioembolization; PD-1: Programmed cell death 1; PD-L1: Programmed cell death-ligand 1; FGFR2: Fibroblast growth factor receptor 2; IDH1: Isocitrate dehydrogenase-1; HER2: Human epidermal growth factor receptor 2; NTRK: Neurotropic tyrosine kinase receptor; RET: Rearranged during transfection; Gem: Gemcitabine; Cis: Cisplatin; Gemox: Gemcitabine and oxaliplatin; FOLFOX: Oxaliplatin, leucovorin, and 5-fluorouracil; ALPPS: Associating liver partition and portal vein ligation for staged hepatectomy; PVE: Portal vein embolization.
- Citation: Liu JJ, Zhou M, Yuan T, Huang ZY, Zhang ZY. Conversion treatment for advanced intrahepatic cholangiocarcinoma: Opportunities and challenges. World J Gastroenterol 2025; 31(15): 104901
- URL: https://www.wjgnet.com/1007-9327/full/v31/i15/104901.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i15.104901