Copyright
©The Author(s) 2025.
World J Gastroenterol. Apr 14, 2025; 31(14): 105004
Published online Apr 14, 2025. doi: 10.3748/wjg.v31.i14.105004
Published online Apr 14, 2025. doi: 10.3748/wjg.v31.i14.105004
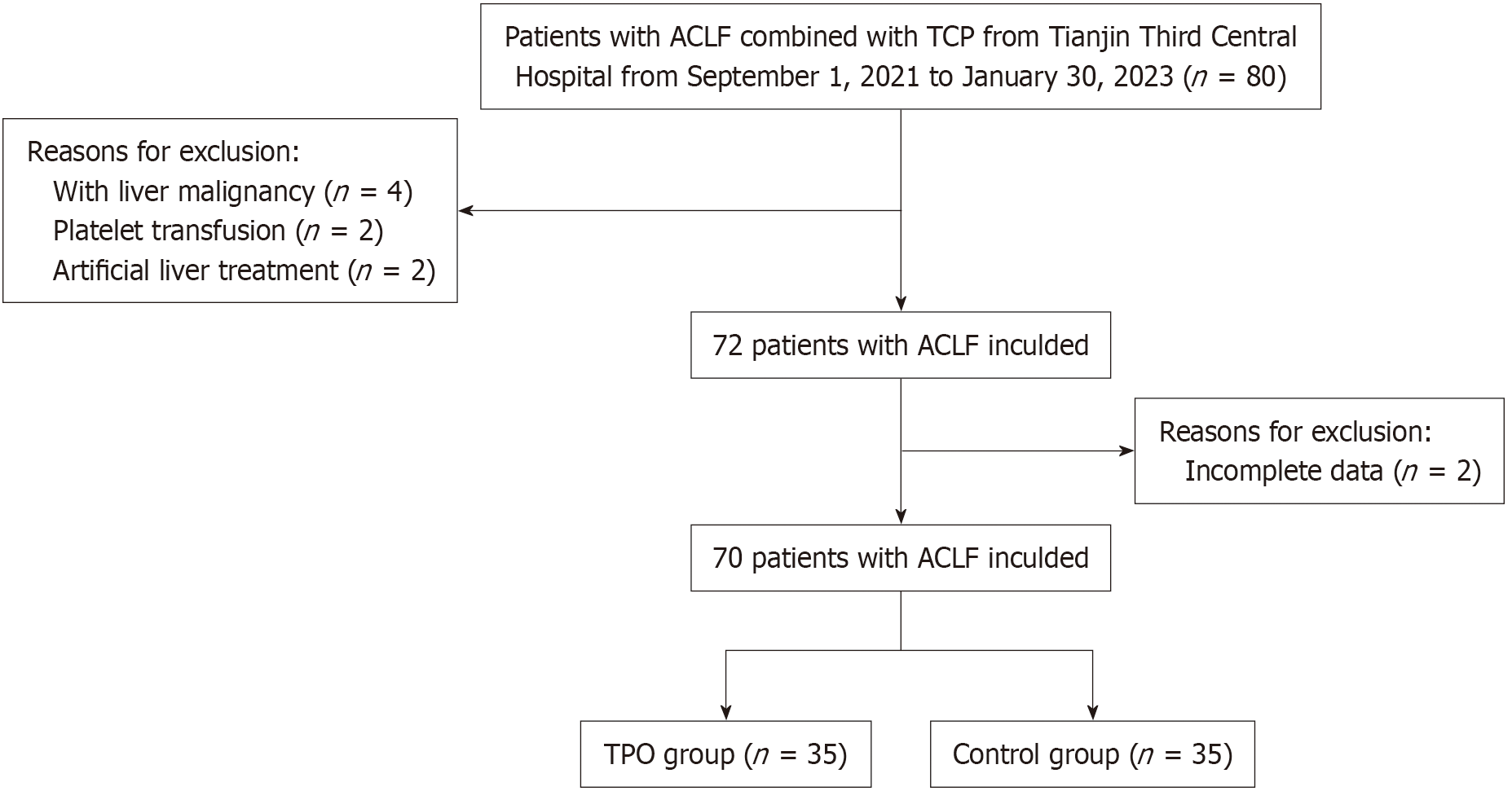
Figure 1 Flow diagram of the population enrolled.
The experimental design flow of this study, including the steps of sample collection, grouping and treatment. Each box represents a stage of the experiment, and the arrows indicate the direction of the process. ACLF: Acute-on-chronic liver failure; TCP: Thrombocytopenia; TPO: Thrombopoietin.
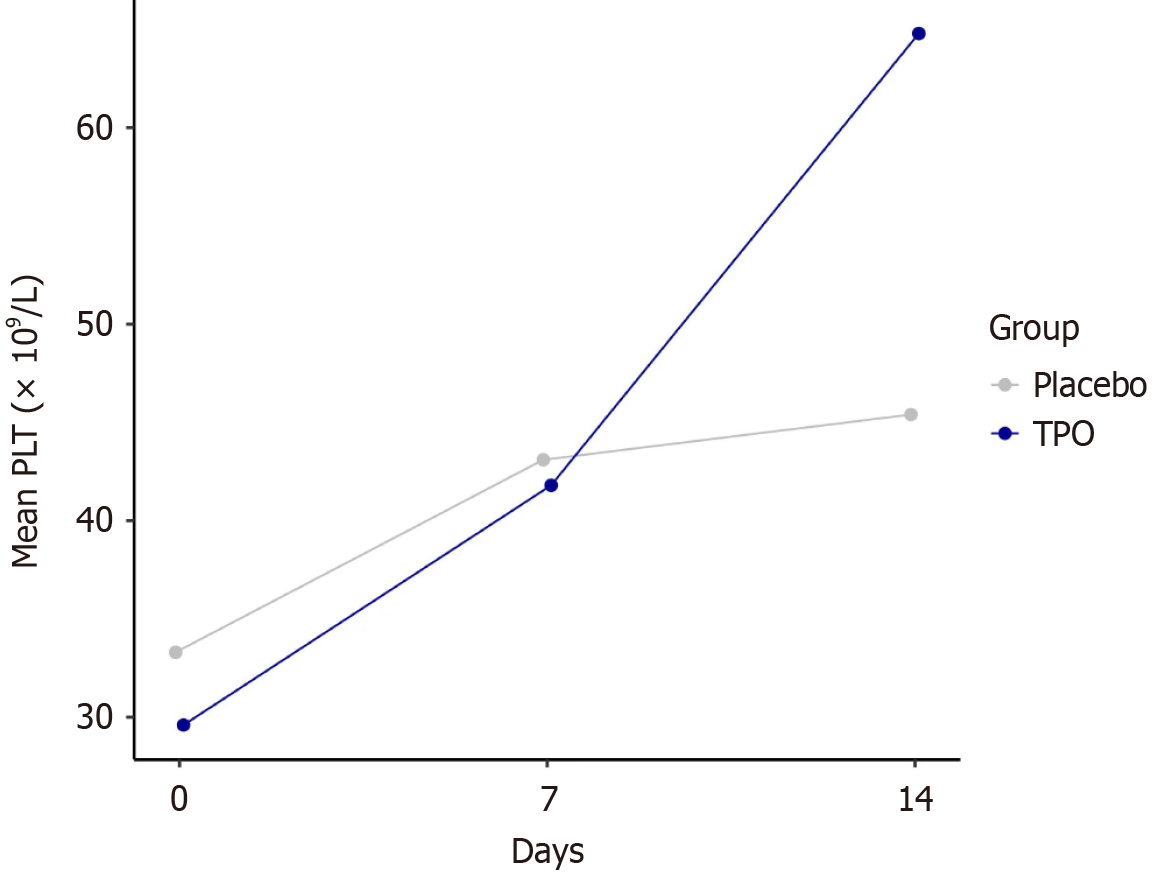
Figure 2 Dynamics of platelet count from baseline to day 14 after recombinant human thrombopoietin treatment.
Compare the platelet count between the two groups at different time points (day 0, day 7, and day 14 of recombinant human thrombopoietin treatment). PLT: Platelet count; TPO: Thrombopoietin.
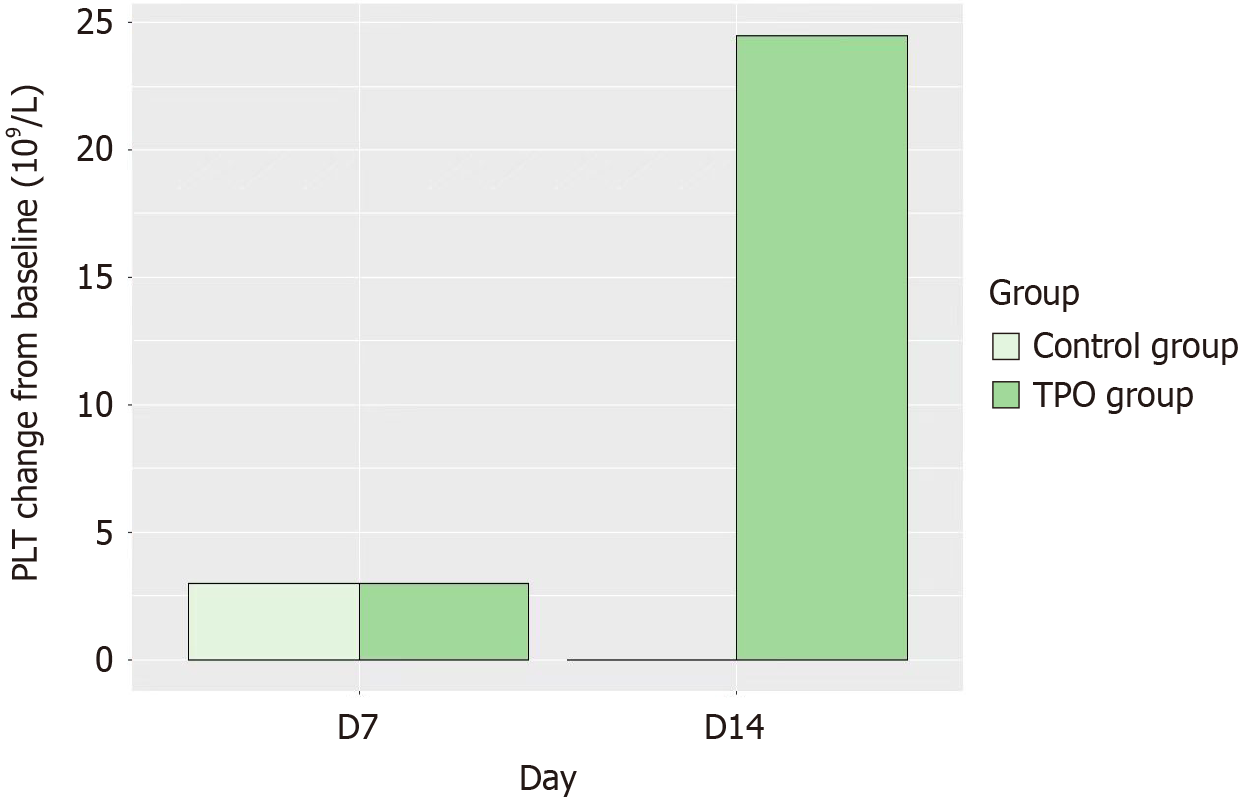
Figure 3 Platelet count change from baseline on day 7 and 14 in the recombinant human thrombopoietin group and control group.
PLT: Platelet count; TPO: Thrombopoietin.
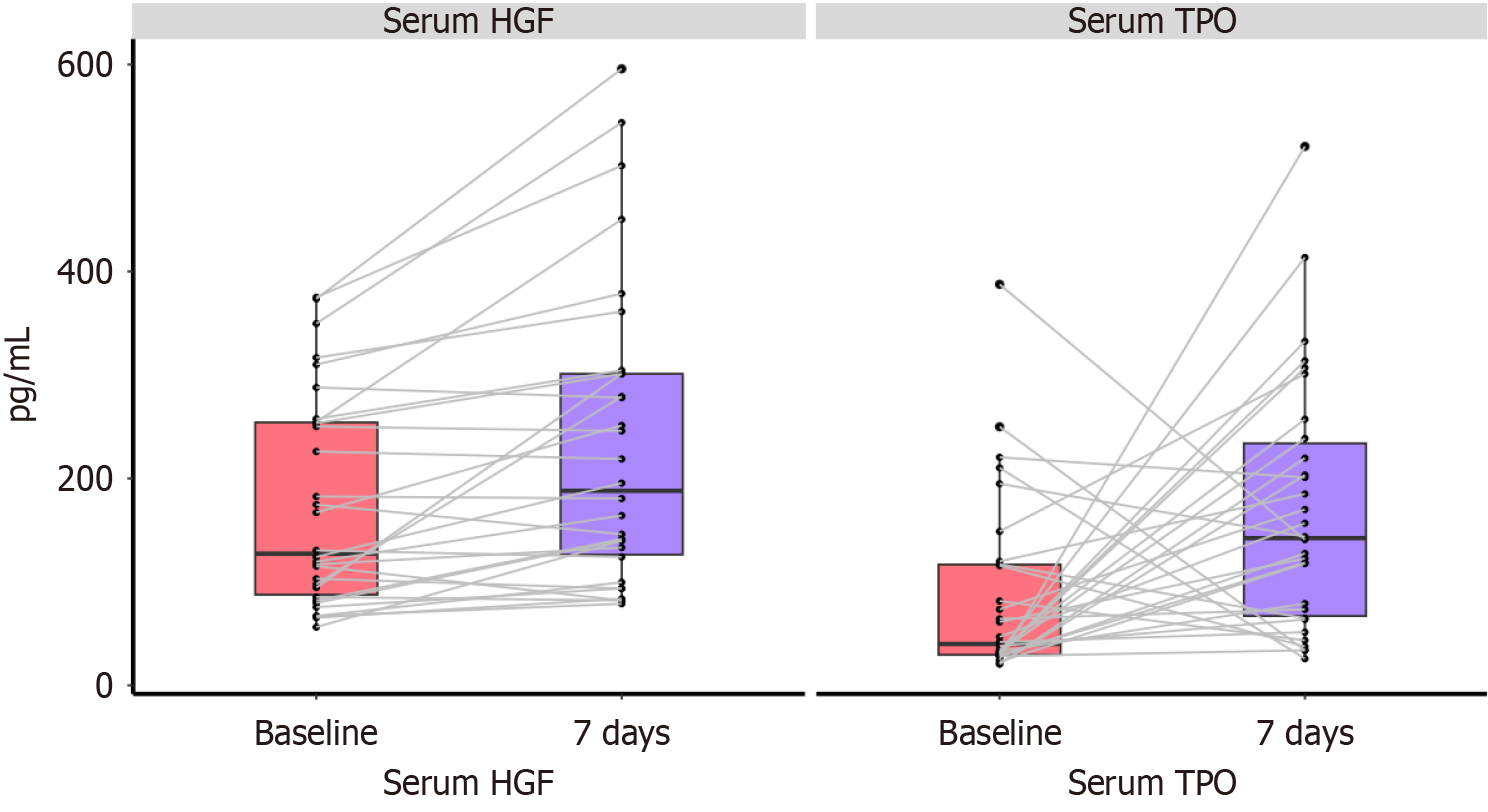
Figure 4 Serum hepatocyte growth factor and thrombopoietin levels before and after treatment in the recombinant human thrombopoietin group.
Serum levels of hepatocyte growth factor and thrombopoietin (TPO) in patients of the recombinant human TPO (rhTPO) group were measured using enzyme-linked immunosorbent assay before treatment and on day 7 of rhTPO treatment. HGF: Hepatocyte growth factor; TPO: Thrombopoietin.
- Citation: Liu G, Tang F, Wang T, Yan JQ, Li FH, Ha FS, Zhang X, Jing L, Liang J. Efficacy of recombinant human thrombopoietin in patients with acute-on-chronic liver failure and thrombocytopenia: A prospective, open-label study. World J Gastroenterol 2025; 31(14): 105004
- URL: https://www.wjgnet.com/1007-9327/full/v31/i14/105004.htm
- DOI: https://dx.doi.org/10.3748/wjg.v31.i14.105004