Copyright
©The Author(s) 2024.
World J Gastroenterol. Sep 7, 2024; 30(33): 3823-3836
Published online Sep 7, 2024. doi: 10.3748/wjg.v30.i33.3823
Published online Sep 7, 2024. doi: 10.3748/wjg.v30.i33.3823
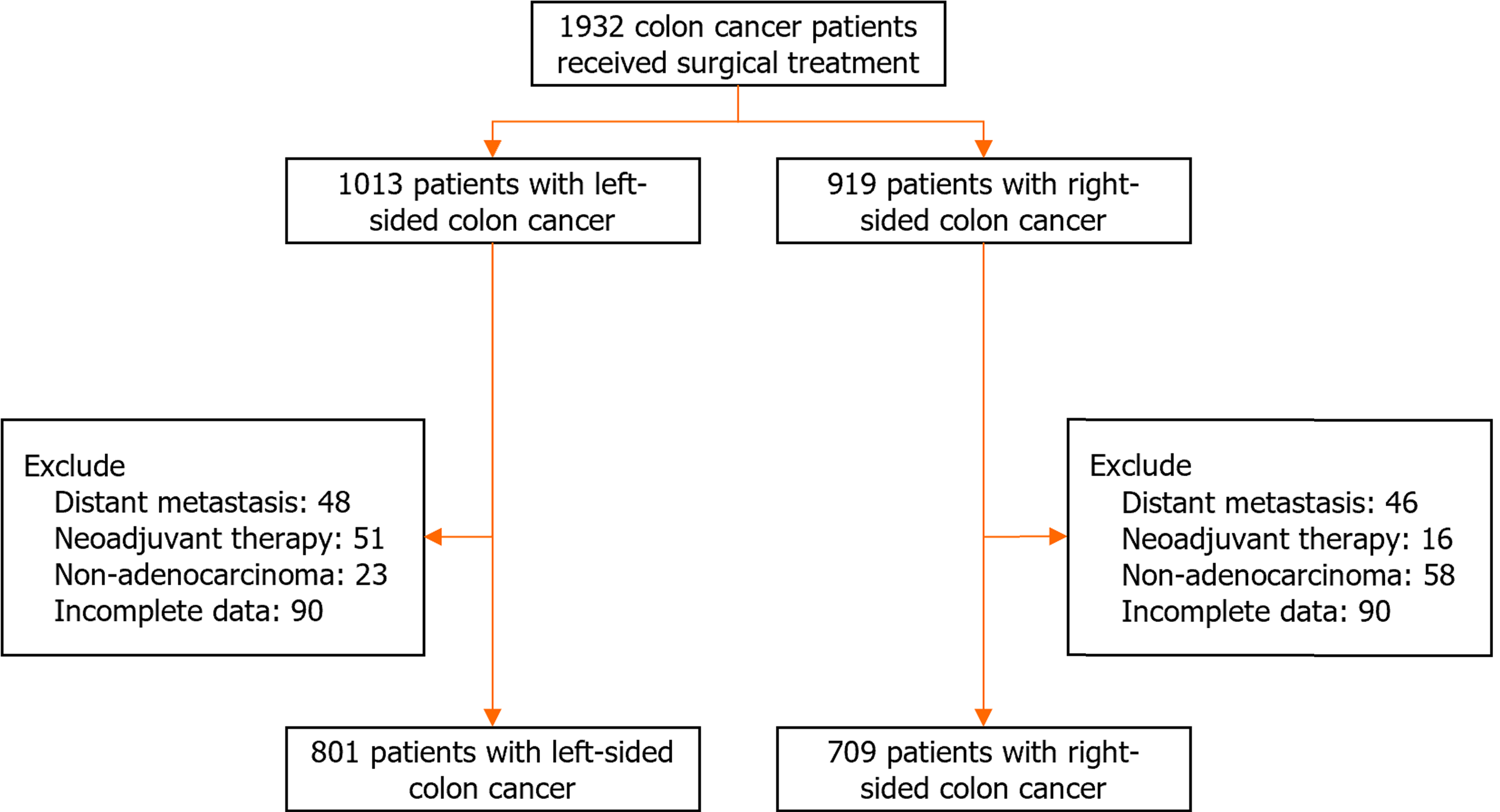
Figure 1 Flowchart for cohort selection with inclusion and exclusion criteria.
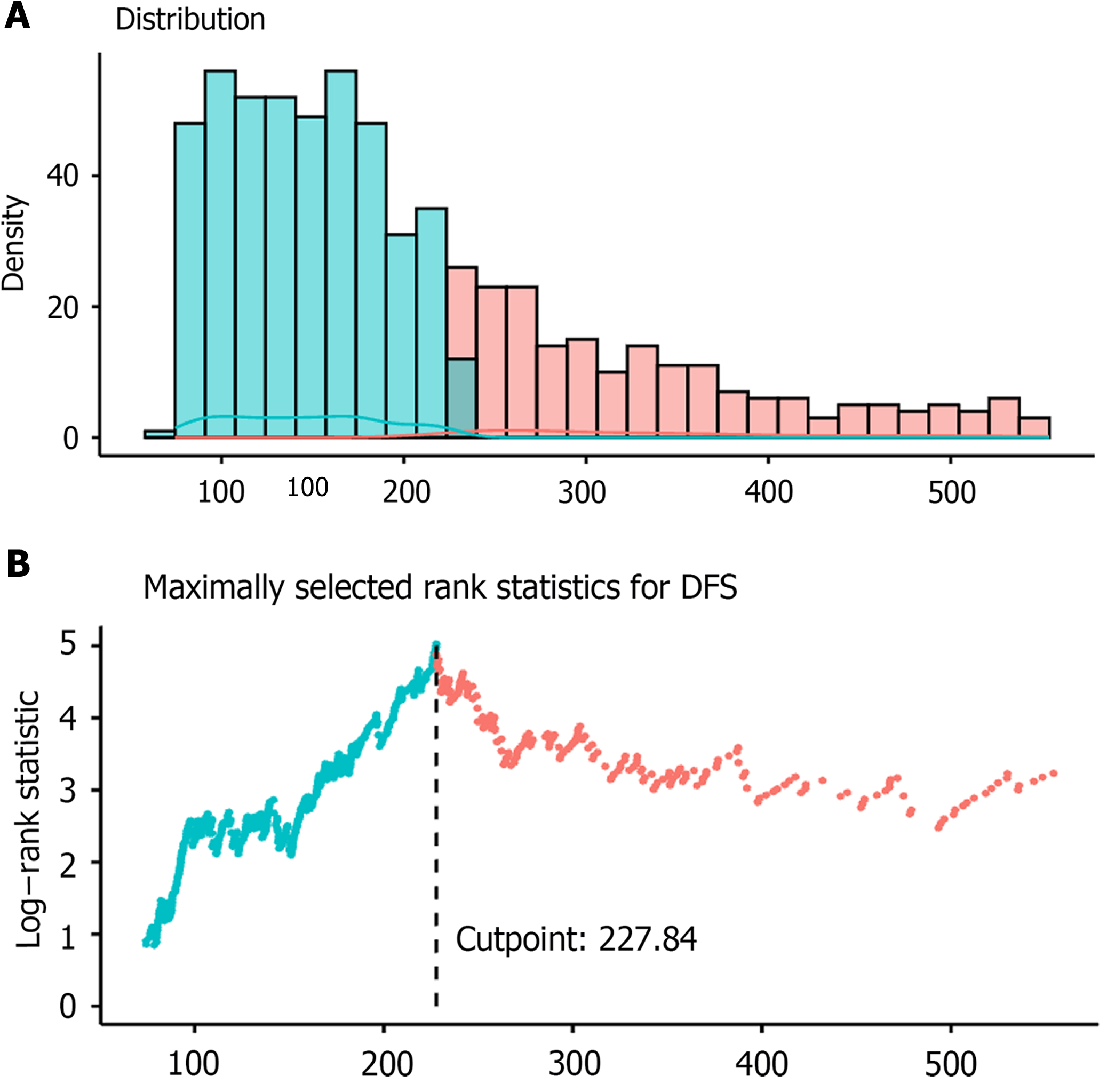
Figure 2 Distribution of pan-immune-inflammation value and threshold evaluation.
A: Distribution of pan-immune-inflammation value; B: Threshold evaluation using maximum log-rank test statistic. DFS: Disease-free survival.
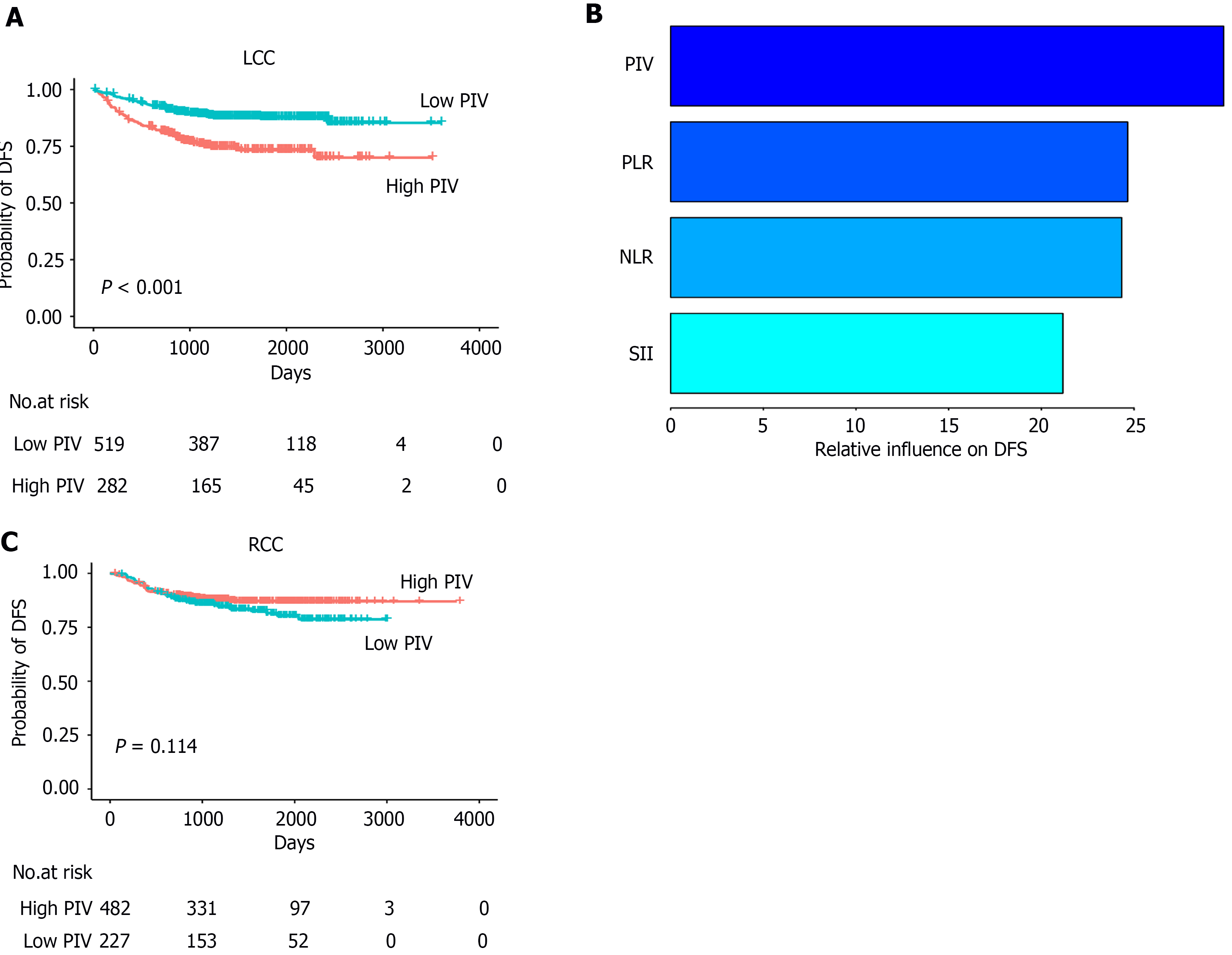
Figure 3 The relationship between pan-immune-inflammation value and disease-free survival in patients with left-sided colon cancer and right-sided colon cancer.
A: In the left-sided colon cancer group, pan-immune-inflammation value (PIV)-high patients had a worse disease-free survival (DFS) than that of PIV-low patients; B: The generalized boosted regression model evaluated the relative impact of systemic inflammatory response markers on DFS, with PIV showing the highest relative influence among systemic inflammatory response markers in left-sided colon cancer patients; C: In the right-sided colon cancer group, there was no significant difference in DFS between PIV-high and -low. LCC: Left-sided colon cancer; RCC: Right-sided colon cancer; PIV: Pan-immune-inflammation value; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; SII: Systemic immune-inflammation index; DFS: Disease-free survival.
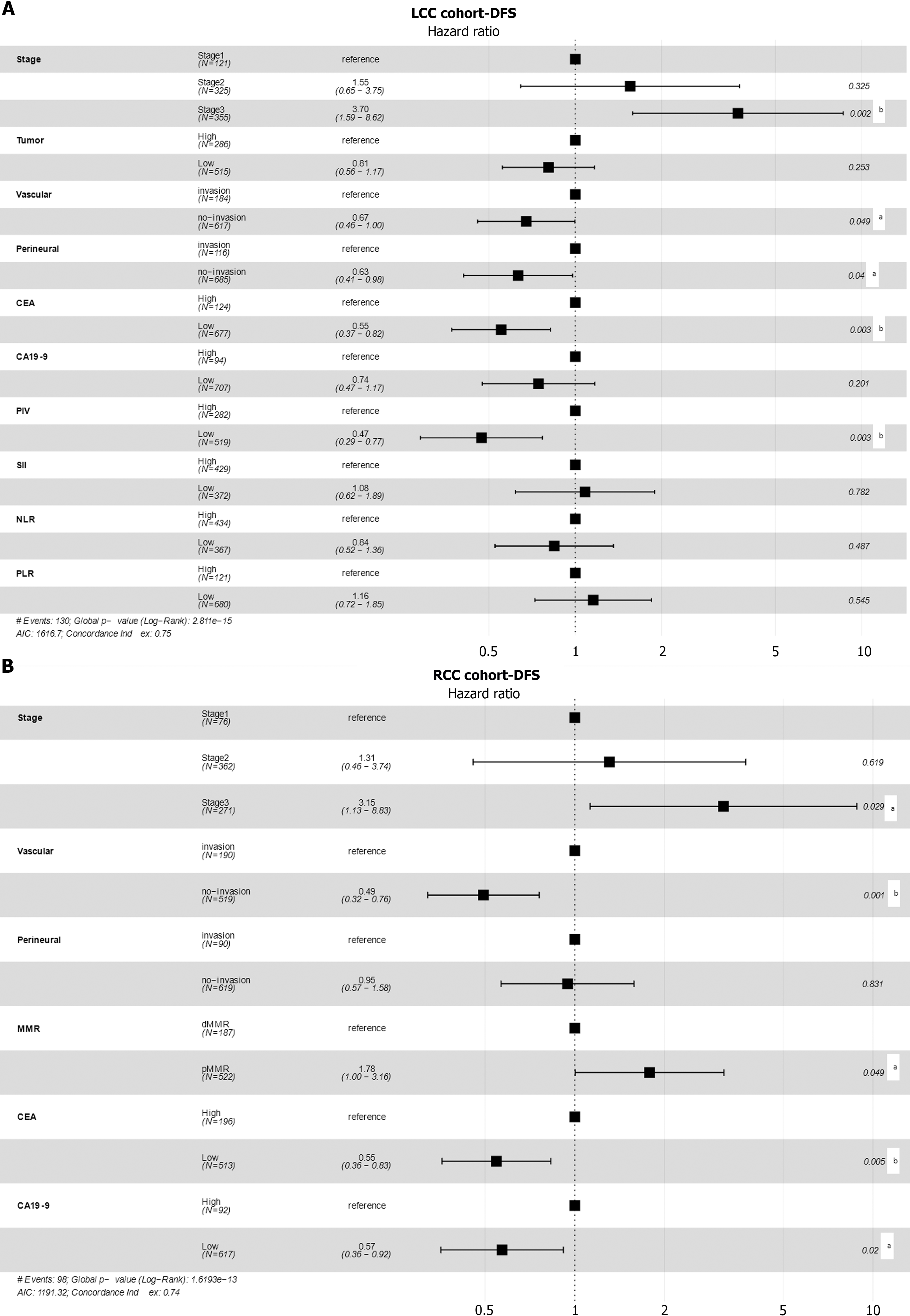
Figure 4 The forest plots for clinicopathological factors in disease-free survival-based multivariate Cox models.
A: The hazard ratios, as well as the 95% confidence intervals and statistical significances, in disease-free survival-based multivariate Cox models for the left-sided colon cancer cohort; B: The hazard ratios, as well as the 95% confidence intervals and statistical significances, in disease-free survival-based multivariate Cox models for the right-sided colon cancer cohort. aP < 0.05; bP < 0.01. LCC: Left-sided colon cancer; RCC: Right-sided colon cancer; DFS: Disease-free survival; PIV: Pan-immune-inflammation value; CA19-9: Carbohydrate antigen 19-9; CEA: Carcinoembryonic antigen; MMR: Mismatch repair; NLR: Neutrophil-to-lymphocyte ratio; PLR: Platelet-to-lymphocyte ratio; SII: Systemic immune-inflammation index.
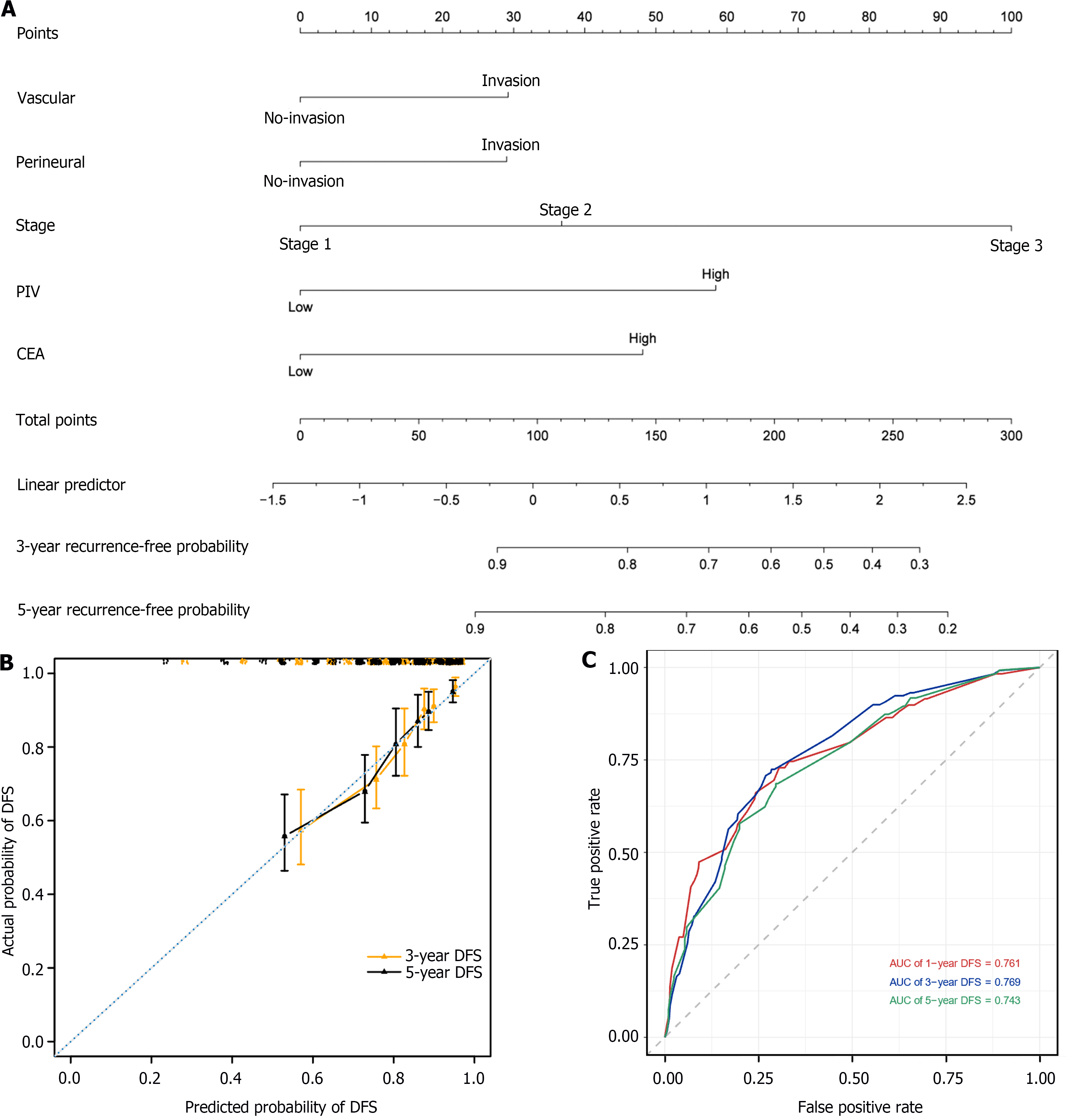
Figure 5 Construction and validation of a nomogram based on multivariate Cox regression analysis.
A: The nomogram integrating vascular invasion, perineural invasion, stage, pan-immune-inflammation value, and carcinoembryonic antigen for the disease-free survival (DFS) prediction of patients with left-sided colon cancer; B: Calibration curves for predicting DFS at 3-year and 5-year time points in left-sided colon cancer patients. The x-axis indicates the predictive survival probabilities by the nomogram, while the y-axis indicates the actual survival probabilities; the 45° dotted line indicates ideal prediction; C: 1-, 3-, and 5-year area under the curves DFS. DFS: Disease-free survival; PIV: Pan-immune-inflammation value; CEA: Carcinoembryonic antigen; AUC: Area under the receiver operating characteristic curve.
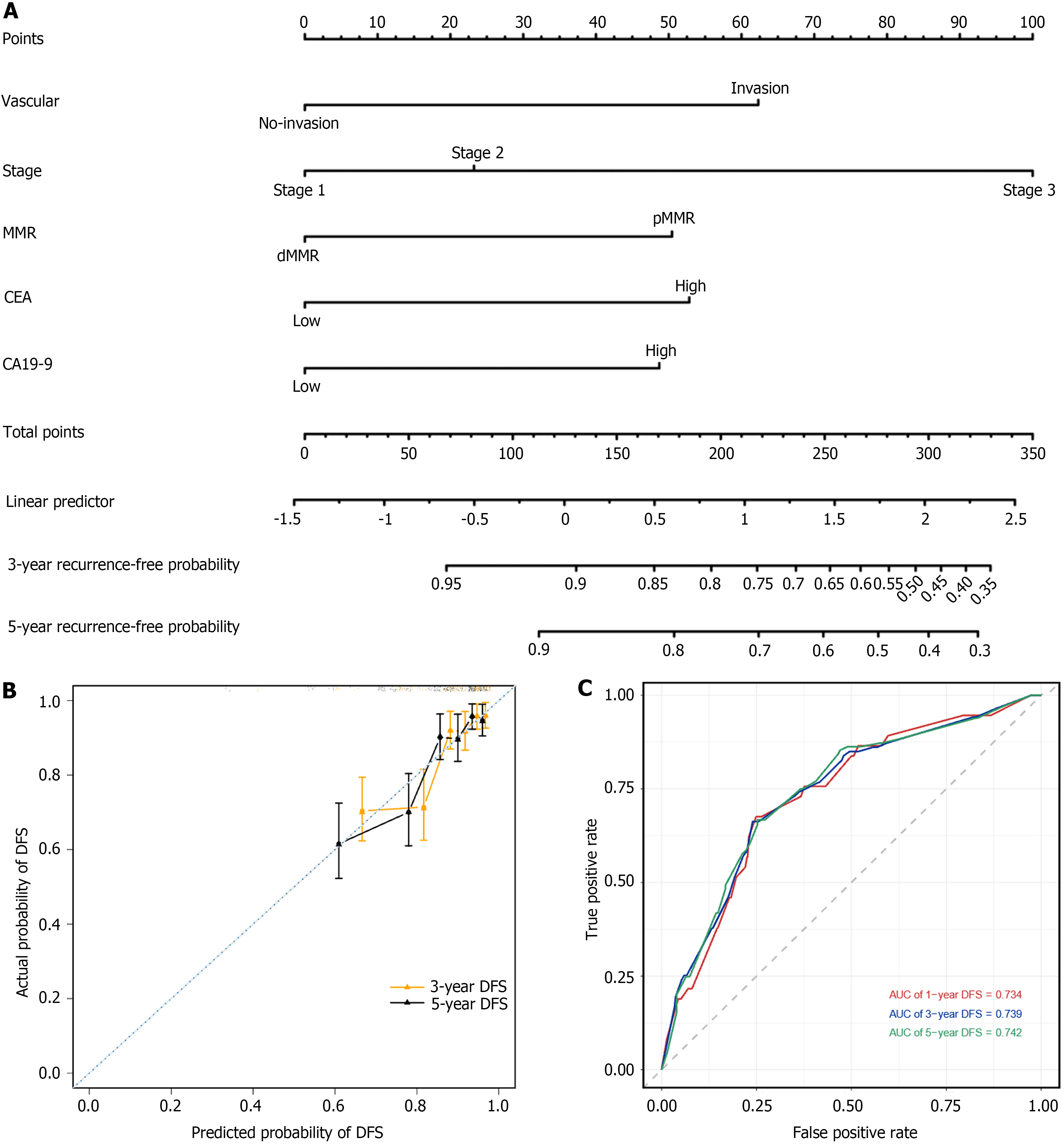
Figure 6 Construction and validation of a nomogram based on multivariate Cox regression analysis.
A: The nomogram integrating vascular invasion, perineural invasion, stage, pan-immune-inflammation value, and carcinoembryonic antigen for the disease-free survival (DFS) prediction of patients with right-sided colon cancer; B: Calibration curves for predicting DFS at 3-year and 5-year time points in right-sided colon cancer patients. The x-axis indicates the predictive survival probabilities by the nomogram, while the y-axis indicates the actual survival probabilities; the 45° dotted line indicates ideal prediction; C: 1-, 3-, and 5-year area under the curves DFS. DFS: Disease-free survival; CA19-9: Carbohydrate antigen 19-9; CEA: Carcinoembryonic antigen; dMMR: Mismatch repair-deficient; pMMR: Mismatch repair-proficient.
- Citation: Wang QY, Zhong WT, Xiao Y, Lin GL, Lu JY, Xu L, Zhang GN, Du JF, Wu B. Pan-immune-inflammation value as a prognostic biomarker for colon cancer and its variation by primary tumor location. World J Gastroenterol 2024; 30(33): 3823-3836
- URL: https://www.wjgnet.com/1007-9327/full/v30/i33/3823.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i33.3823