Copyright
©The Author(s) 2024.
World J Gastroenterol. Aug 28, 2024; 30(32): 3766-3782
Published online Aug 28, 2024. doi: 10.3748/wjg.v30.i32.3766
Published online Aug 28, 2024. doi: 10.3748/wjg.v30.i32.3766
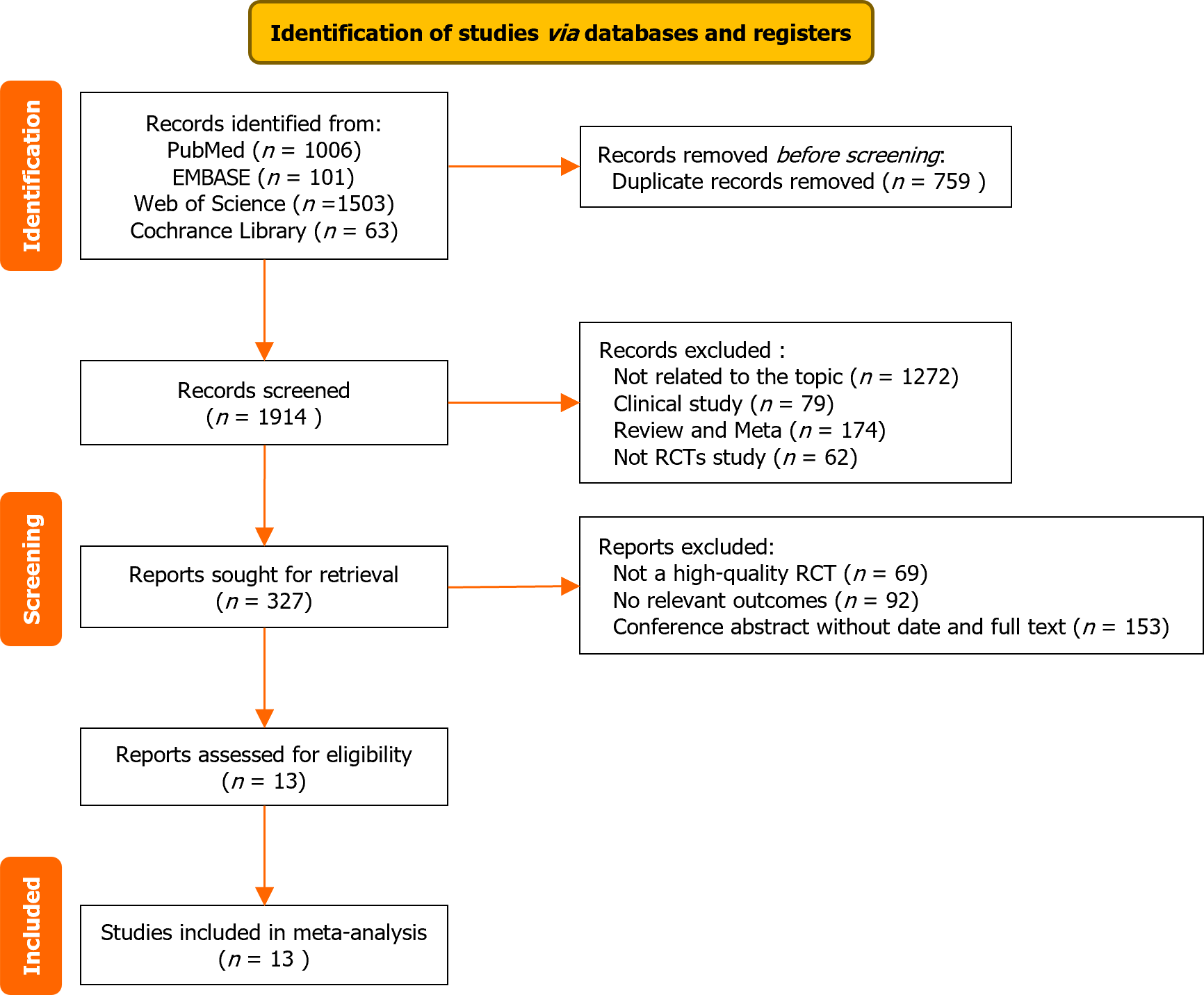
Figure 1 Literature selection and inclusion process.
RCT: Randomized control trial.
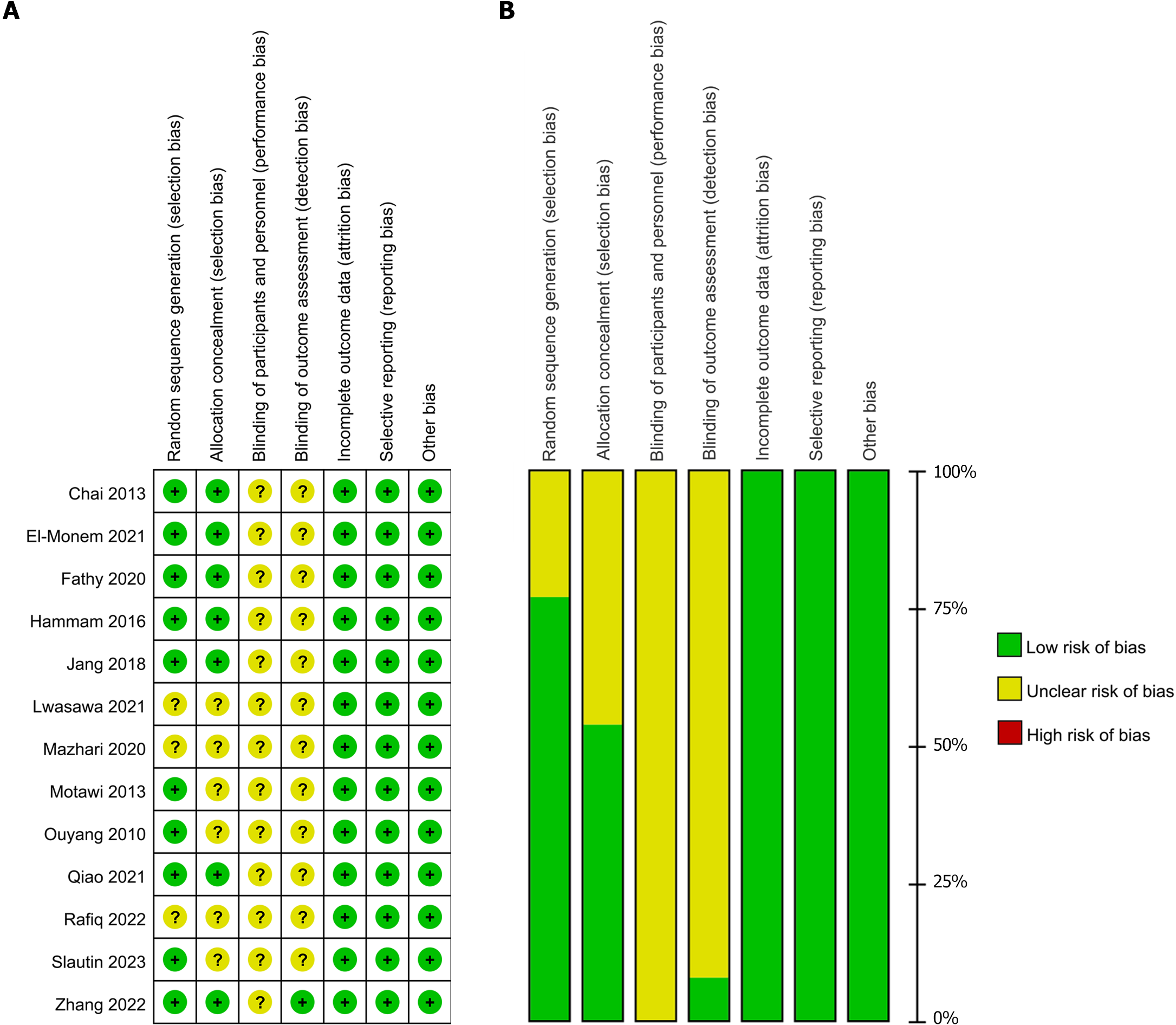
Figure 2 Risk of bias graph and bias summary.
A: Review authors’ judgments about each risk of bias item presented as percentages across all included studies; B: Review authors’ judgments about each risk of bias item for each included study.
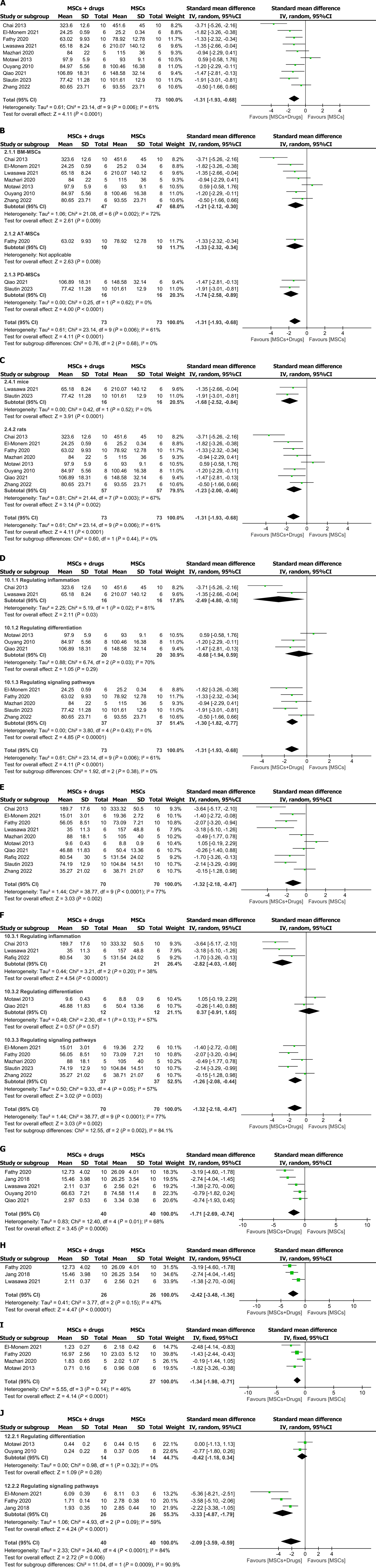
Figure 3 Forest plots.
A: Aspartate aminotransferase (AST); B: Mesenchymal stem cells source subgroups of AST levels; C: Animal model subgroup of AST levels; D: Mechanism subgroup of AST levels; E: Alanine aminotransferase (ALT); F: Mechanism subgroup of ALT levels; G and H: Hydroxyproline; I: Forest plot of type III collagen; J: Mechanism subgroup of transforming growth factor beta gene levels. MSCs: Mesenchymal stem cells; 95%CI: 95% confidence interval; BM-MSCs: Bone marrow mesenchymal stem cells; AT-MSCs: Adipose mesenchymal stem cells; PD-MSCs: Placental mesenchymal stem cells.
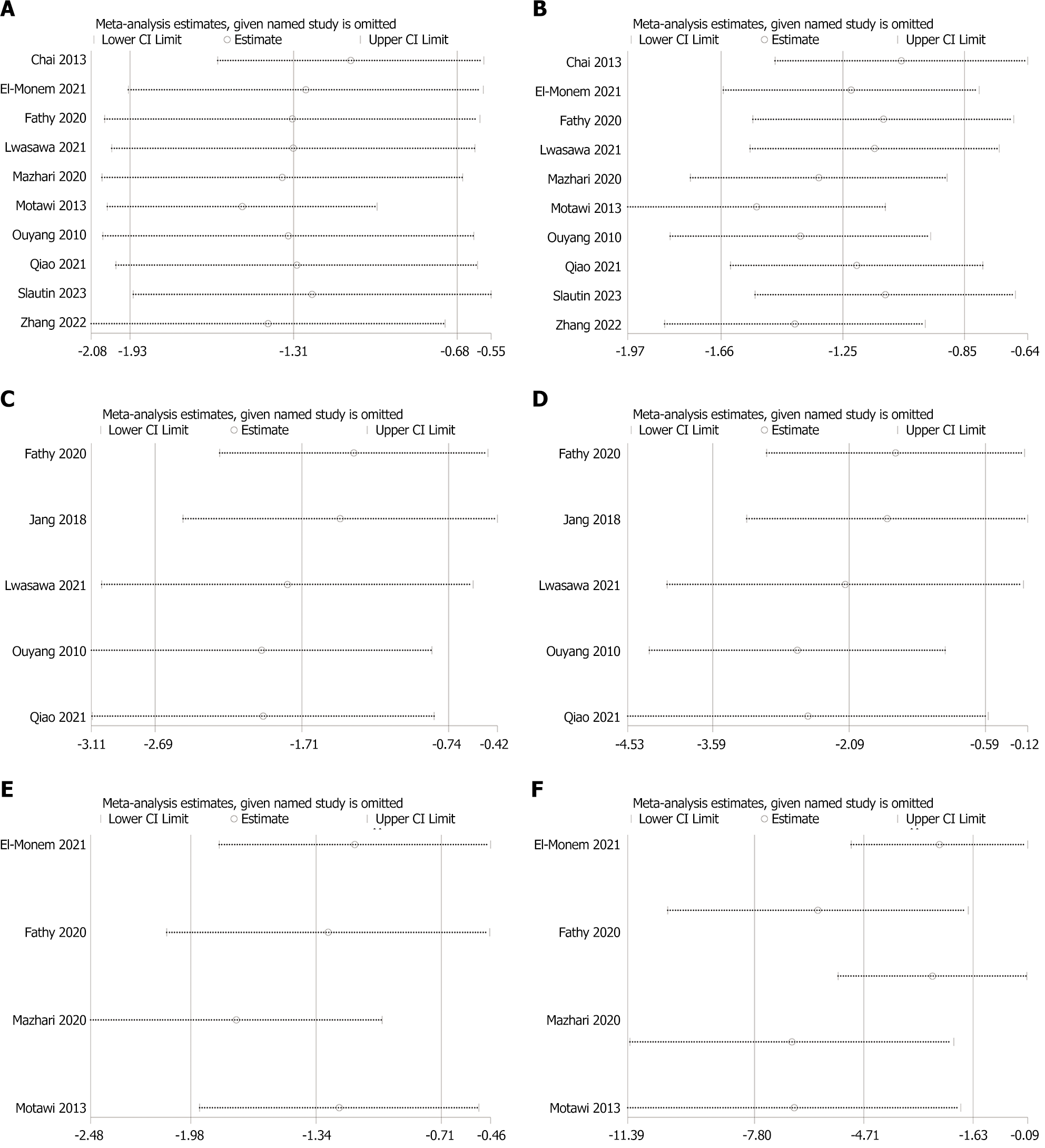
Figure 4 Sensitivity analysis.
A: Aspartate aminotransferase; B: Alanine aminotransferase; C: Hydroxyproline; D: Transforming growth factor-β; E: Type Ⅲ collagen; F: Masson staining.
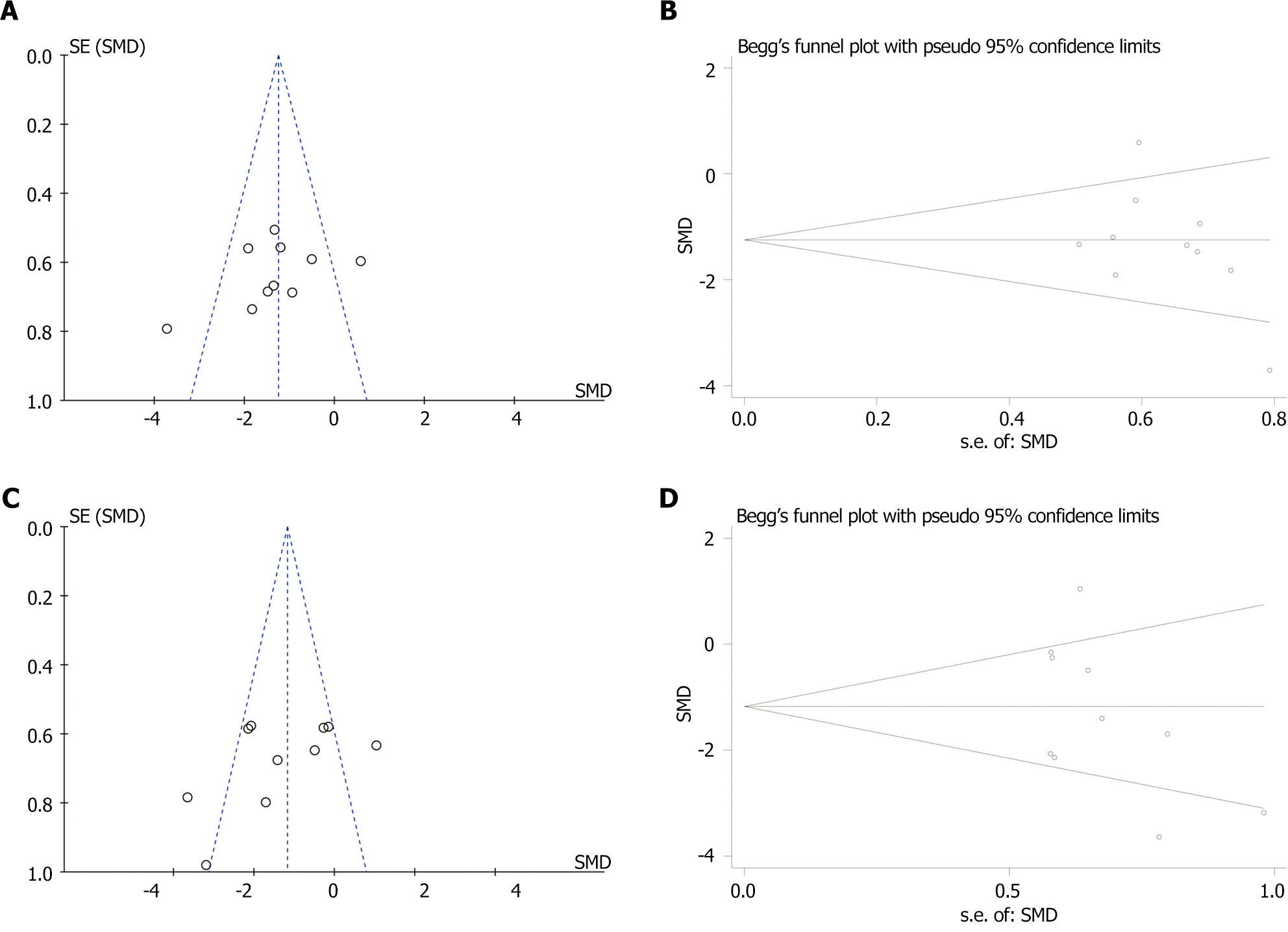
Figure 5 Funnel plot and Egger’s publication bias plot.
A: Funnel plot with pseudo-95% confidence limits [aspartate aminotransferase (AST)]; B: Egger’s publication bias plot (AST); C: Funnel plot with pseudo-95% confidence limits [alanine aminotransferase (ALT)]; D: Egger’s publication bias plot (ALT). SMD: Standardized mean difference.
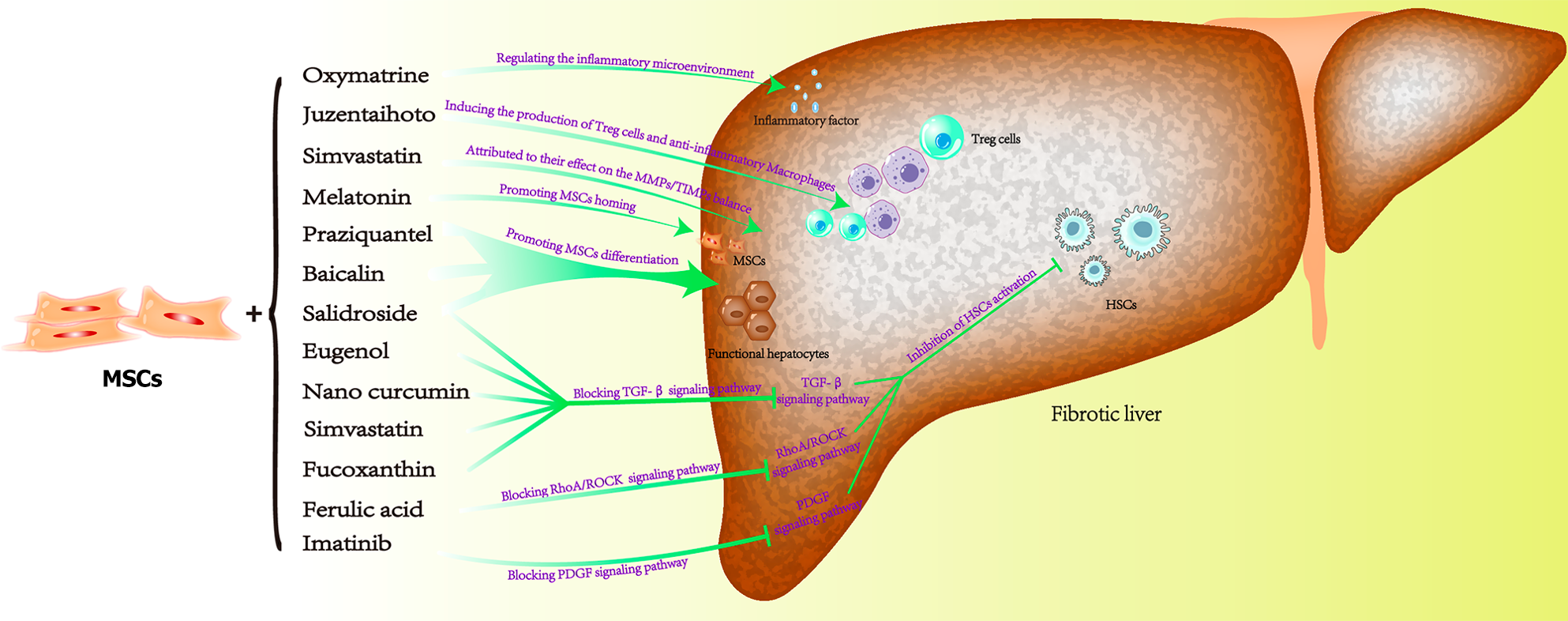
Figure 6 Diagram of the mechanism of mesenchymal stem cell therapy for liver fibrosis.
MSCs: Mesenchymal stem cells; HSCs: Hepatic stellate cells.
- Citation: Xu Y, Wang XS, Zhou XL, Lu WM, Tang XK, Jin Y, Ye JS. Mesenchymal stem cell therapy for liver fibrosis need “partner”: Results based on a meta-analysis of preclinical studies. World J Gastroenterol 2024; 30(32): 3766-3782
- URL: https://www.wjgnet.com/1007-9327/full/v30/i32/3766.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i32.3766