Copyright
©The Author(s) 2024.
World J Gastroenterol. Jan 21, 2024; 30(3): 268-279
Published online Jan 21, 2024. doi: 10.3748/wjg.v30.i3.268
Published online Jan 21, 2024. doi: 10.3748/wjg.v30.i3.268
Figure 1 Cholera toxin induces diarrhea but does not alter calcium-sensing receptor in the intestine.
A: Representative images of fluid accumulation in the intestine; B: Representative images of fluid accumulation in the cecum; C: Quantifications of fluid accumulation in the intestine; D: Representative western blot for intestinal calcium-sensing receptor (CaSR); E: Quantification of intestinal CaSR western blot. Mice were intragastrically gavaged with vehicle or cholera toxin at the indicated doses to induce diarrhea/intestinal fluid secretion. Three and half-hours later, animals were killed, intestines removed, and fluid and CaSR protein quantitated. The CaSR protein signals were normalized by heat shock protein 90 as an internal control to correct protein loading differences. Shown are means ± standard error. aP < 0.05, bP < 0.01 vs no cholera toxin control. The blue color noted in intestines was Evans Blue dye that was used to monitor intestinal motility (data not shown; will be reported separately). CTX: Cholera toxin; HSP90: Heat shock protein 90.
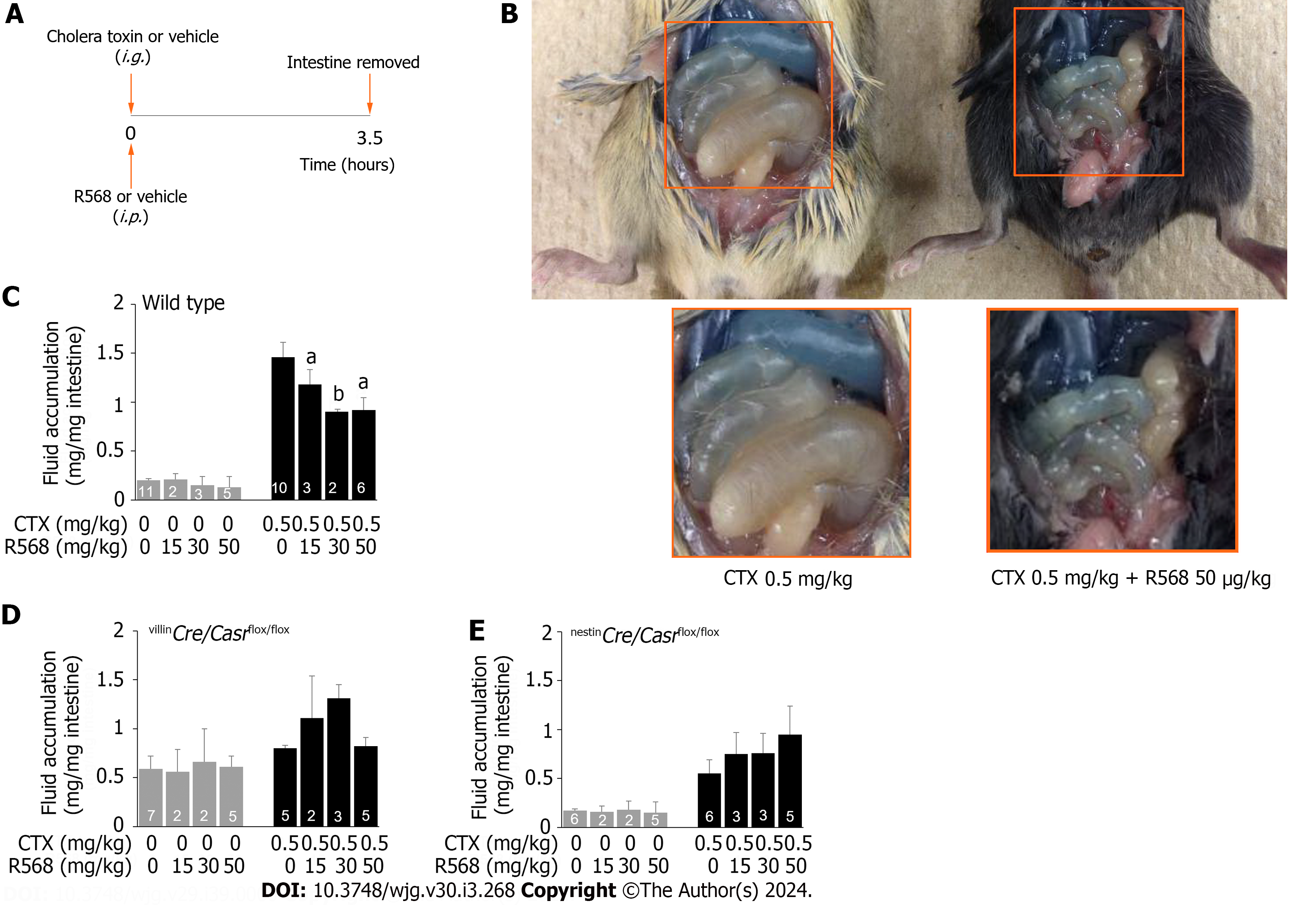
Figure 2 Calcimimetic R568 reverses cholera toxin-induced diarrhea in wild type but not in intestine-specific calcium-sensing receptor knockouts.
A: Experimental protocol; B: Representative images of R568 effect on fluid accumulation in the intestine of wild type mice; C: Summary of fluid accumulation in the intestine of wild type mice; D: Summary of fluid accumulation in the intestine of villinCre/Casrflox/flox mice; E: Summary of fluid accumulation in the intestine of nestinCre/Casrflox/flox mice. Mice were intragastrically gavaged with cholera toxin or vehicle while receiving R568 or vehicle intraperitoneally. Three and half-hours later, animals were killed, intestines removed, and fluid quantitated. Shown are means ± standard errors. aP < 0.05, bP < 0.01 vs without R568. CTX: Cholera toxin.
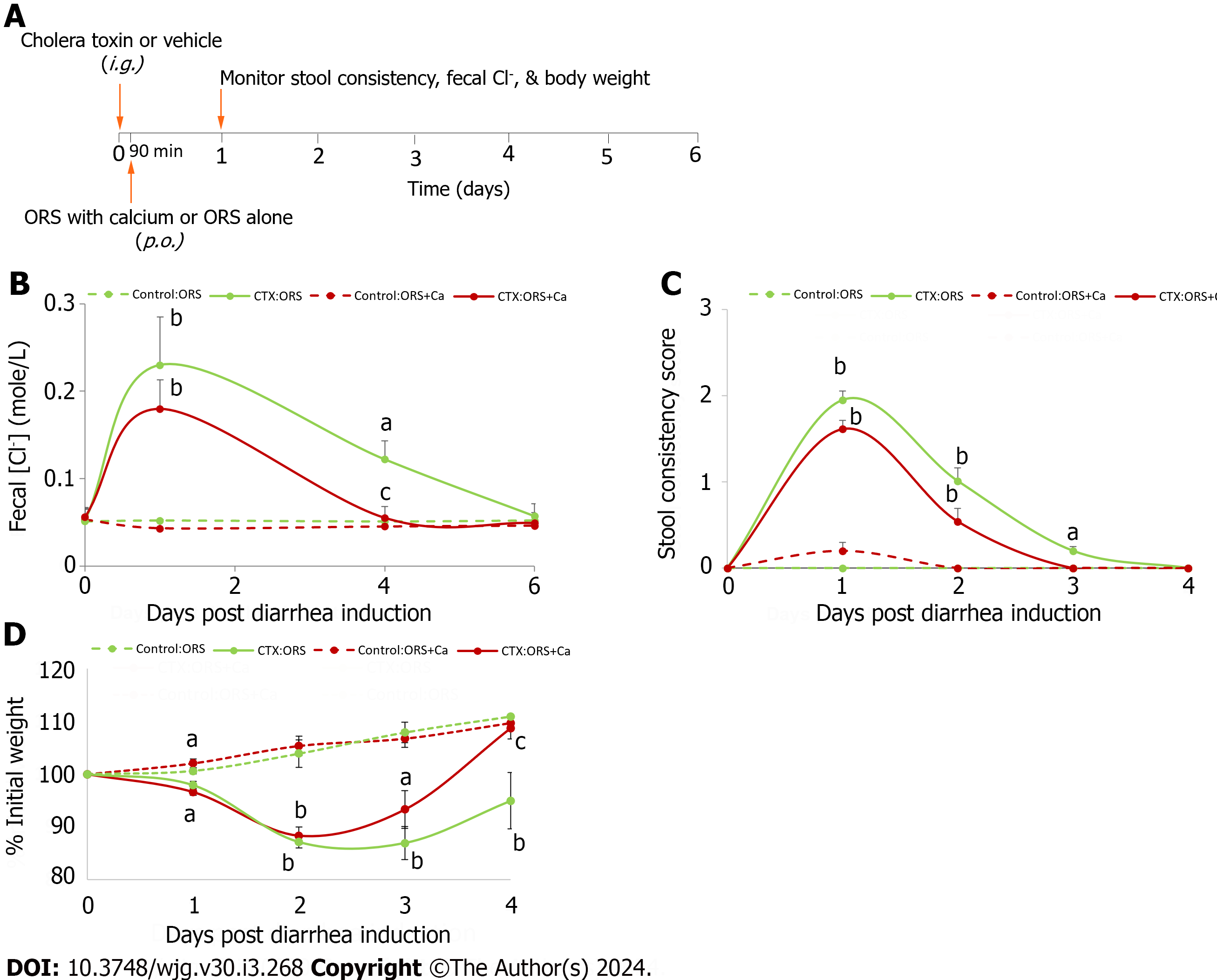
Figure 3 Adding calcium to oral rehydration solution rescues cholera toxin-induced intestinal Cl- loss and stool consistency, and promotes the rate of rehydration.
A: Experimental protocol; B: Changes in fecal Cl-; C: Changes in stool consistency score; D: Changes in % initial weight. At day 0, all animals were intragastrically gavaged with 20 μg/mouse of cholera toxin (CTX) to induce diarrhea. Ninety minutes later, they were allowed to drink oral rehydration solution (ORS), or ORS supplemented with 5 mmol/L calcium (ORS + Ca). Changes in fecal Cl- (B), stool consistency score (C) and % initial weight (D) were monitored. Shown are means ± standard errors. n = 5-6 for each data point. No significant differences between groups were noted in initial body weights (in grams): Control:ORS vs ORS + Ca: 19.1 ± 0.6 vs 19.1 ± 0.6; CTX:ORS vs ORS + Ca: 19.9 ± 0.6 vs 19.5 ± 0.4. aP < 0.05, bP < 0.01 vs no cholera toxin controls; cP < 0.05 vs oral rehydration solution control. ORS: Oral rehydration solution; CTX: Cholera toxin.
Figure 4 Diagram illustrates how cholera toxin stimulates and calcium/calcimimetic inhibits the dual pathways of Cl- secretion resulting in diarrhea.
Cholera toxin (CTX) via Gs α-subunit stimulates transepithelial Cl- secretion both neurally through Nestin-expressing enteric neurons releasing neurotransmitters and non-neuronally through direct action on Villin-expressing epithelial cells. The outcome is stimulating both Cl- entry from blood side into intestinal epithelial cell and exit from epithelial cell into the intestinal lumen causing secretory diarrhea. On the other hand, Ca2+/calcimimetic via calcium-sensing receptor (CaSR) inhibits both actions of CTX via direct epithelial action and indirectly via enteric neuron. The involvement of Nestin-expressing enteric neurons and Villin-expressing epithelial cells is evidenced in the two tissue-specific knockout mice whose Cl- secretory responses to CTX and CaSR stimulation are compromised.
- Citation: Tang LQ, Fraebel J, Jin S, Winesett SP, Harrell J, Chang WH, Cheng SX. Calcium/calcimimetic via calcium-sensing receptor ameliorates cholera toxin-induced secretory diarrhea in mice. World J Gastroenterol 2024; 30(3): 268-279
- URL: https://www.wjgnet.com/1007-9327/full/v30/i3/268.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i3.268