Copyright
©The Author(s) 2024.
World J Gastroenterol. Jun 21, 2024; 30(23): 2964-2980
Published online Jun 21, 2024. doi: 10.3748/wjg.v30.i23.2964
Published online Jun 21, 2024. doi: 10.3748/wjg.v30.i23.2964
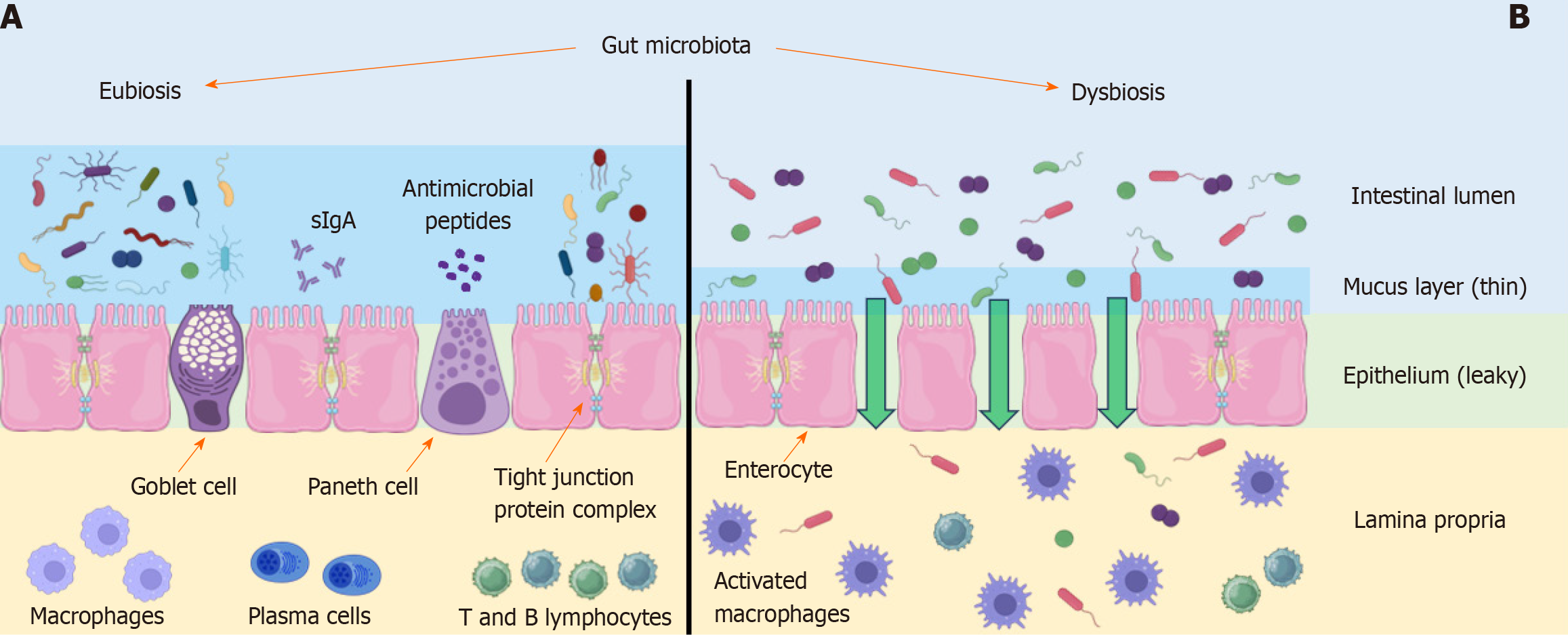
Figure 1 Intestinal barrier in healthy and metabolic dysfunction-associated fatty liver disease syndrome people.
Comparison of the intestinal barrier and mechanisms involved in its disruption. A: Healthy people; B: Metabolic dysfunction-associated fatty liver disease (MAFLD) syndrome people. The composition of the gut microbiota plays a critical role in maintaining the integrity of the intestinal barrier through several mechanisms: regulation of mucus layer thickness produced by goblet cells, production of antimicrobial peptides by Paneth cells, levels of tight junction proteins responsible for epithelial cell integrity, and the activation of immune cells such as macrophages, plasma cells, and T and B lymphocytes. These mechanisms are located in different compartments of the intestinal barrier. In individuals with MAFLD syndrome, dysbiosis results in a reduction in mucous layer thickness, antimicrobial peptides production, and the levels of tight junction proteins. There is also an alteration in the number of immune cells in the lamina propria. Macrophage activation leads to the production of pro-inflammatory cytokines. These factors contribute to increased intestinal permeability and disturbance of the gut-liver axis homeostasis. The figure was created using Servier Medical Art application offered by Servier and licensed under a Creative Commons Attribution 3.0 Unported License.
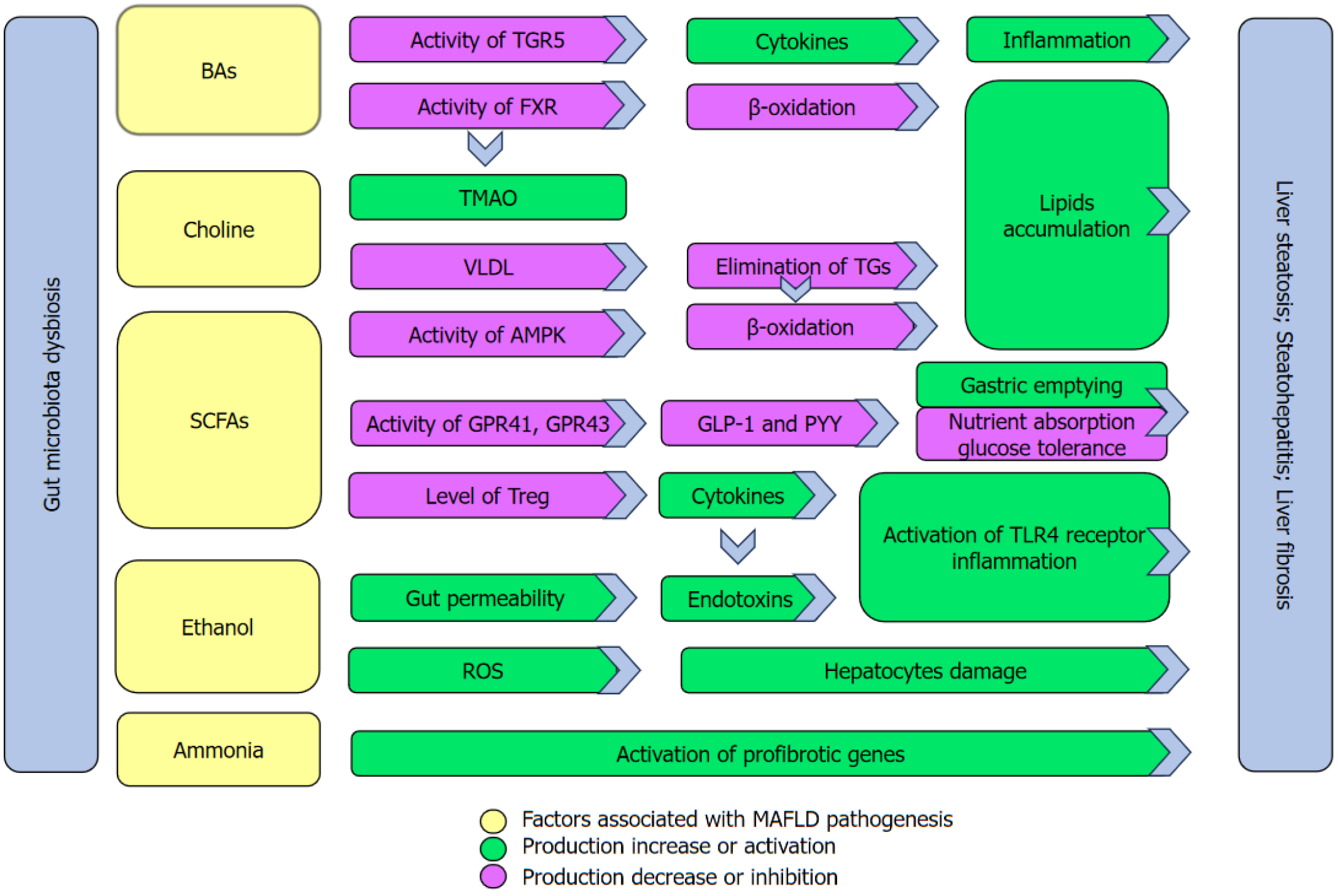
Figure 2 Key mechanisms associated with metabolite disruption in metabolic dysfunction-associated fatty liver disease progression.
Gut microbiota dysbiosis in patients with metabolic dysfunction-associated fatty liver disease causes alterations of important intestinal lumen metabolites. Changes in bile acid composition lead to a decrease in the activity of Takeda G protein-coupled receptor 5 (TGR5) and Farnesoid X receptor (FXR) in the liver. TGR5 is responsible for suppressing pro-inflammatory cytokines and its reduction promotes inflammatory processes in the liver. This contributes markedly to liver fibrosis. Decreased FXR activity leads to a decline in β-oxidation processes that promotes the accumulation of lipids in the liver. Trimethylamino-N-oxide (TMAO) is a product of choline metabolism that is modified by gut microbiota and liver enzymes and is involved in the reduction of FXR activity. Increased TMAO levels reduce the bioavailability of choline in the liver that is involved in the synthesises of very-low-density lipoprotein (VLDL). Lowered VLDL levels reduce the elimination of triglycerides that promotes liver steatosis. A reduction in short-chain fatty acid levels is responsible for: (1) A decrease in the activity of liver AMP-activated protein kinase that causes a drop in the capacity of β-oxidation processes; (2) a decrease in activity of G-protein receptors (GPR41, GPR43) in intestinal enteroendocrine L cells that leads to reduced production of glucagon-like peptide-1 and peptide YY , leading to a disruption in energy homeostasis; and (3) decreased levels of T-regulatory lymphocytes that play an important role in inhibiting pro-inflammatory cytokines. Ethanol may provoke damage to the intestinal barrier that is associated with increased endotoxins levels in the portal system, ultimately reaching the liver. Activation of toll-like receptor 4 and the inflammasome by endotoxins leads to an increase in the production of pro-inflammatory cytokines that causes hepatic inflammation. Furthermore, ethanol increases the production of reactive oxygen species that results in oxidative damage to hepatocytes. Ammonia is involved in hepatic fibrosis by activating the profibrotic genes in hepatic stellate cells. BA: Bile acid; TGR5: Takeda G protein-coupled receptor 5; FXR: Farnesoid X receptor; TMAO: Trimethylamino-N-oxide; VLDL: Very-low-density lipoprotein; SCFA: Short-chain fatty acid; ROS: Reactive oxygen species; TG: Triglyceride; PYY: Peptide YY; GLP-1: Glucagon-like peptide-1; TLR4: Toll-like receptor 4.
- Citation: Rochoń J, Kalinowski P, Szymanek-Majchrzak K, Grąt M. Role of gut-liver axis and glucagon-like peptide-1 receptor agonists in the treatment of metabolic dysfunction-associated fatty liver disease. World J Gastroenterol 2024; 30(23): 2964-2980
- URL: https://www.wjgnet.com/1007-9327/full/v30/i23/2964.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i23.2964