Copyright
©The Author(s) 2024.
World J Gastroenterol. Jun 21, 2024; 30(23): 2934-2946
Published online Jun 21, 2024. doi: 10.3748/wjg.v30.i23.2934
Published online Jun 21, 2024. doi: 10.3748/wjg.v30.i23.2934
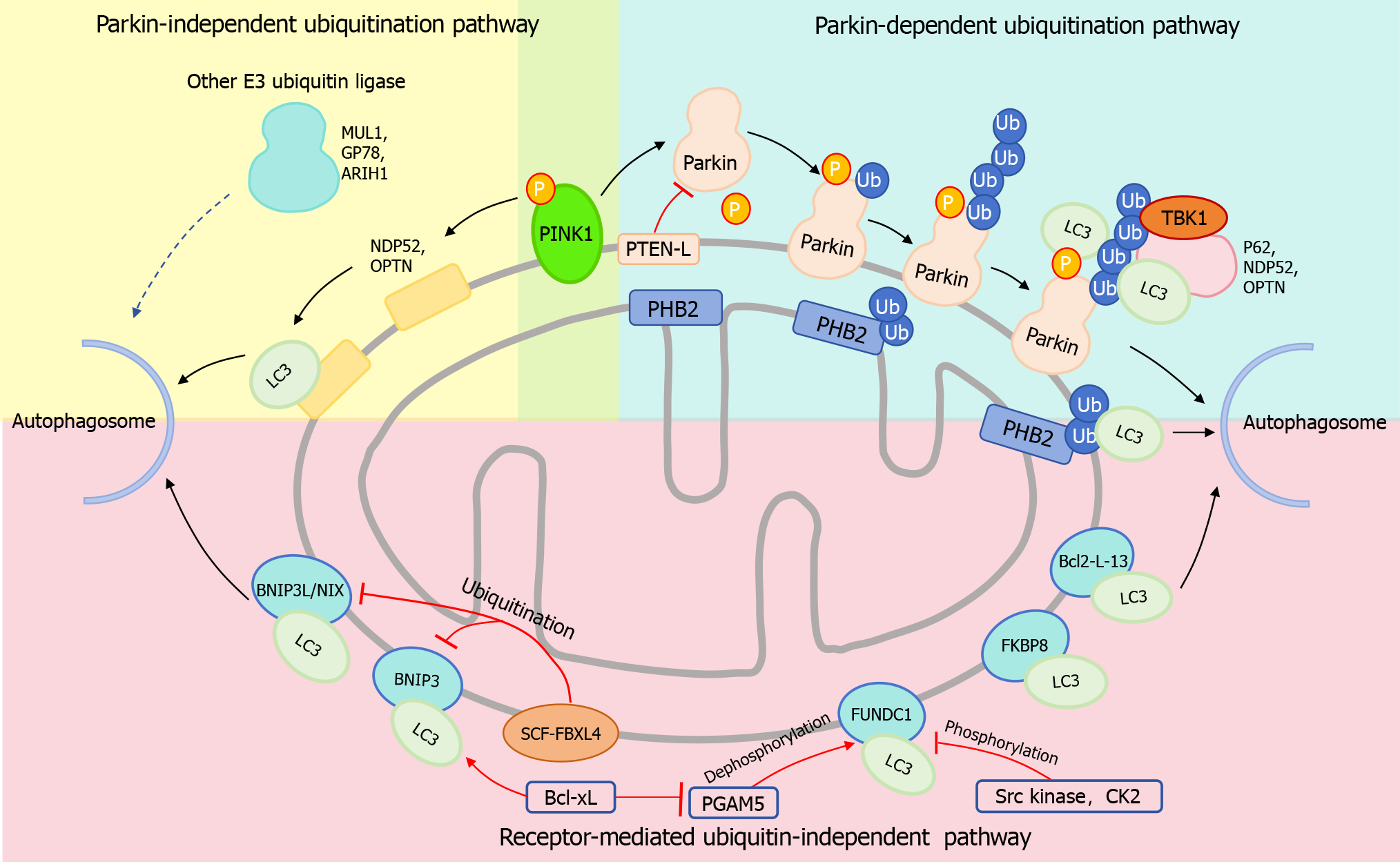
Figure 1 Major Signaling pathways of mitophagy.
Parkin-dependent Ubiquitinated Mitophagy Pathway: Following the depolarization of the mitochondrial membrane, PINK1 recruits and activates the E3 ubiquitin ligase activity of Parkin, leading to the formation of ubiquitin chains. These chains then attract a series of autophagy receptors including P62, NDP52, and OPTN. Subsequently, these receptors bind to light chain 3 (LC3), facilitating the connection of the polyubiquitinated mitochondrial outer membrane to the autophagosome membrane, thereby mediating mitophagy. In this process, TANK-binding kinase 1 kinase enhances the affinity of the autophagy receptor for the Ub chain by phosphorylating the receptor. PTEN-L can reduce Parkin phosphorylation and inhibit its E3 ligase activity, thereby inhibiting mitophagy. Parkin-Independent Ubiquitinated Mitophagy Pathway: E3 ubiquitin ligases such as ARIH1, MUL1, and Gp78 may serve as compensatory pathways for Parkin-mediated mitophagy, although the precise mechanisms are yet to be elucidated. Furthermore, PINK1 has the ability to recruit NDP52 and OPTN to mitochondria, thereby directly initiating mitophagy in a Parkin-independent manner. Receptor-Mediated Ubiquitination-Independent Mitophagy Pathway: Proteins including FK506 binding protein 8, BCL2-interacting protein 3 like/NIP3-like protein X (BNIP3L/NIX), BNIP3, FUN14 domain containing 1 (FUNDC1), and Bcl2-L-13 directly bind to LC3, enabling the mitochondrial membrane to connect to the autophagosome membrane and mediate mitophagy. SCF-FBXL4 mediates the ubiquitination and degradation of BNIP3L/NIX and BNIP3, thereby inhibiting mitophagy. Under hypoxia conditions, phosphoglycerate mutase 5 (PGAM5) promotes the dephosphorylation of FUNDC1, enhancing FUNDC1-mediated mitophagy. Conversely, Src kinase and Casein kinase 2 phosphorylate FUNDC1, inhibiting its mitophagy-promoting activity. Although Bcl-xL positively regulates the binding of BNIP3 to LC3, it inhibits FUNDC1-mediated mitophagy by suppressing PGAM5. LC3: Light chain 3; BNIP3L/NIX: BCL2-interacting protein 3 like/NIP3-like protein X; FUNDC1: FUN14 domain containing 1; TBK1: TANK-binding kinase 1.
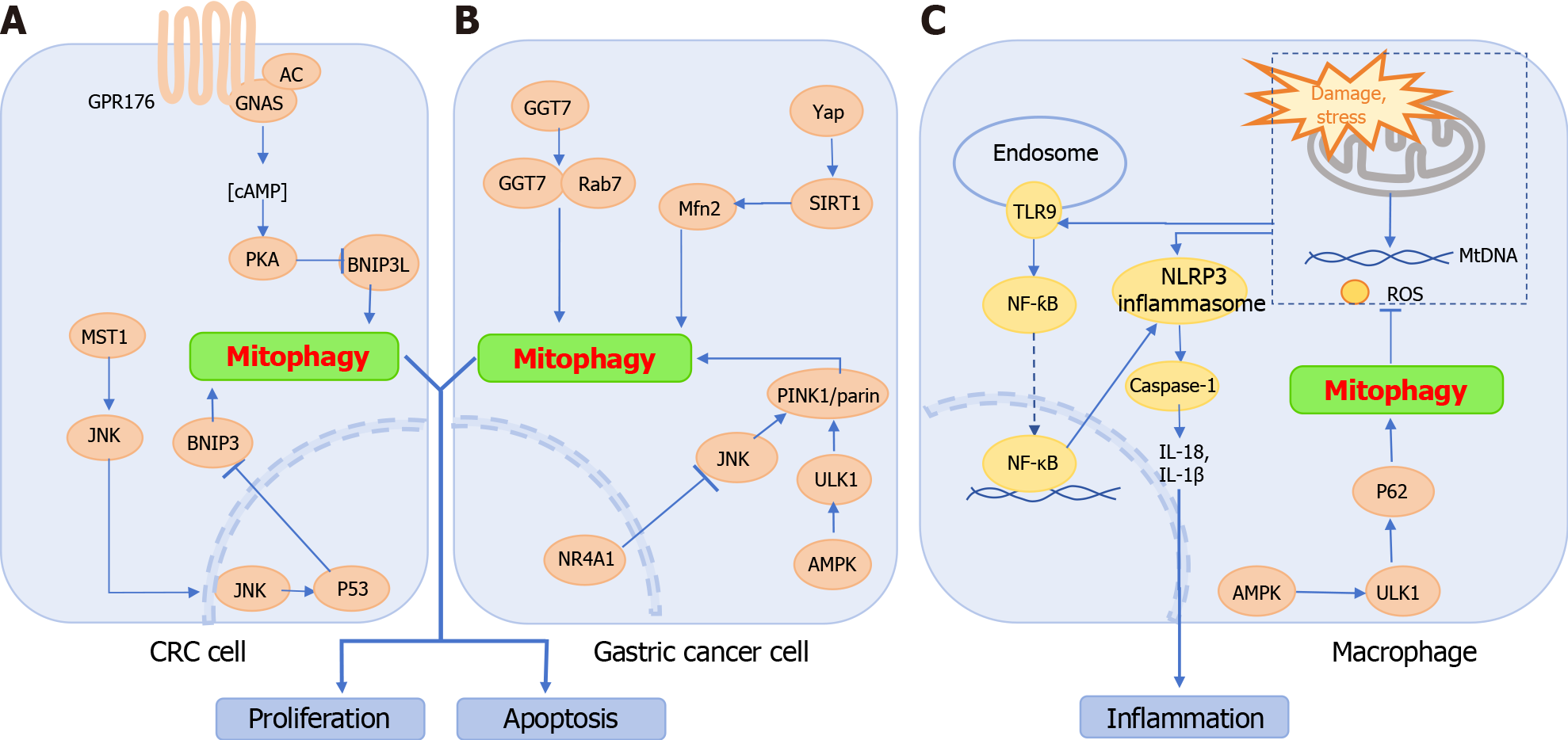
Figure 2 Pathways regulating mitophagy in colorectal cancer, gastric cancer, and inflammatory bowel disease.
A: Colorectal cancer cells: GPR176 recruits GNAS to inhibit BCL2-interacting protein 3 like through the AC/cAMP/PKA pathway, thereby suppressing mitophagy. Additionally, MST1 activates the c-Jun N-terminal kinase (JNK) pathway, up-regulating P53 expression, which in turn inhibits BNIP3 transcription and activity, leading to mitophagy arrest; B: Gastric cancer cells: Interactions between GGT7 and Rab7 promote mitophagy. Yap activates sirtuin 1, enhancing Mfn2 expression and sustaining mitophagy. JNK upregulates Parkin to activate mitophagy; however, overexpression of NR4A1 inhibits mitophagy by suppressing JNK. Furthermore, the AMPK/ULK1/Parkin axis also supports mitophagy; C: Macrophages in inflammatory bowel disease: Damaged or stressed mitochondria in macrophages release mtDNA and reactive oxygen species, which directly contribute to NOD-like receptor thermal protein domain associated protein 3 (NLRP3) inflammasome formation and activate NF-κB via the TLR9 pathway, triggering NLRP3 inflammasome activation and subsequent Caspase-1 activity. This results in the production of IL-1β and IL-18. Enhancing the AMPK-ULK1-P62 axis-driven mitophagy efficiently removes damaged mitochondria, inhibiting NLRP3 inflammasome activation and exerting anti-inflammatory effects. CRC: Colorectal cancer cell; NLRP3: NOD-like receptor thermal protein domain associated protein 3; SIRT1: Sirtuin 1; JNK: c-Jun N-terminal kinase.
- Citation: Gao DL, Lin MR, Ge N, Guo JT, Yang F, Sun SY. From macroautophagy to mitophagy: Unveiling the hidden role of mitophagy in gastrointestinal disorders. World J Gastroenterol 2024; 30(23): 2934-2946
- URL: https://www.wjgnet.com/1007-9327/full/v30/i23/2934.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i23.2934