Copyright
©The Author(s) 2024.
World J Gastroenterol. Apr 7, 2024; 30(13): 1871-1886
Published online Apr 7, 2024. doi: 10.3748/wjg.v30.i13.1871
Published online Apr 7, 2024. doi: 10.3748/wjg.v30.i13.1871
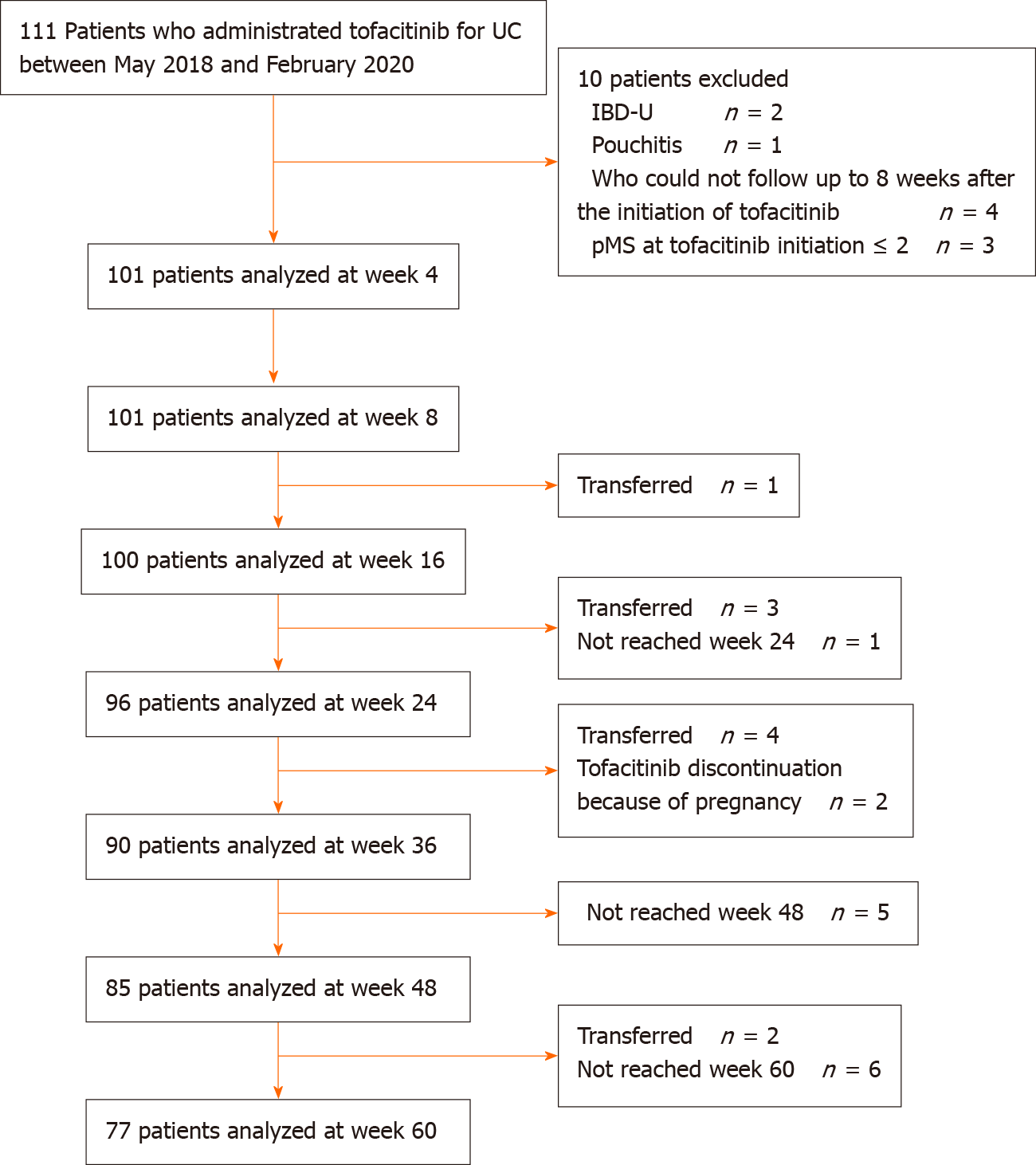
Figure 1 Flow diagram of the participants analyzed.
IBD-U: Inflammatory bowel disease-unclassified; pMS: Partial Mayo Score; UC: Ulcerative colitis.
Figure 2 Rate of clinical response, clinical remission, and steroid-free remission at weeks 4, 8, 16, 24, 36, 48, and 60.
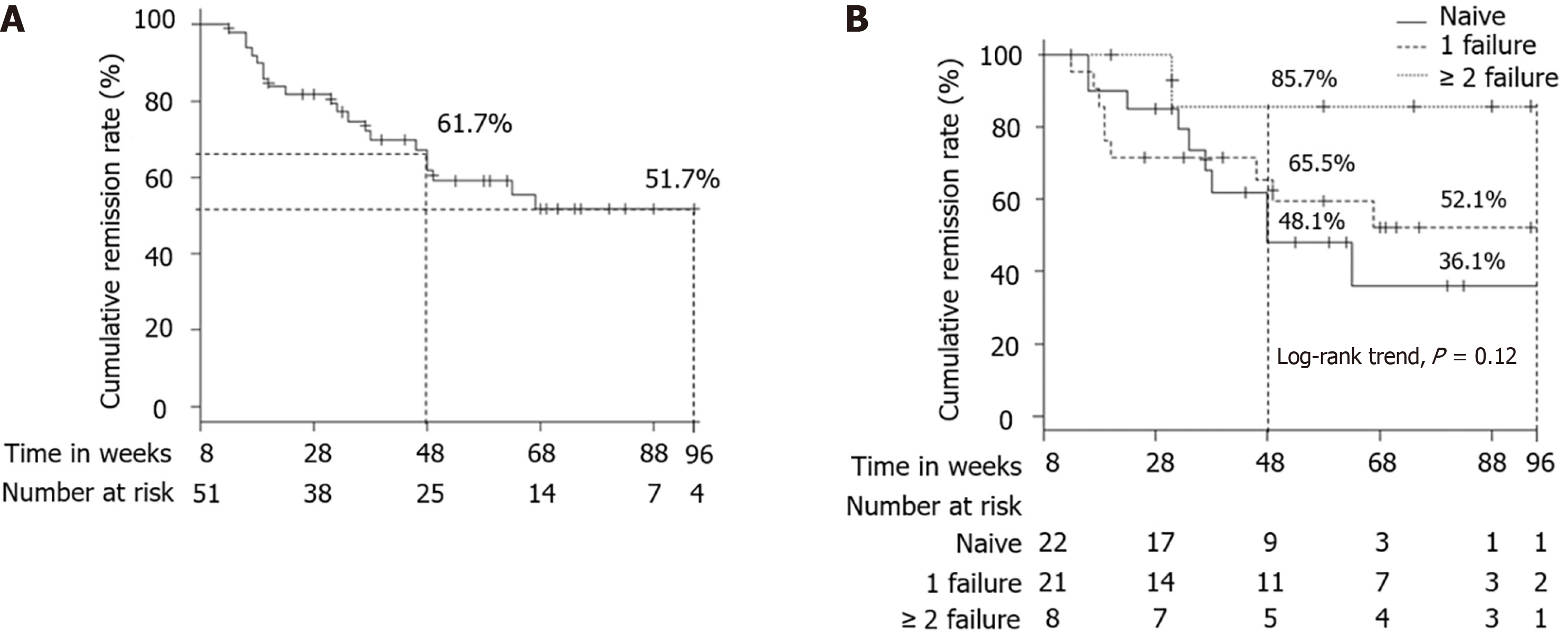
Figure 3 Cumulative remission rate.
A: Cumulative remission rate in patients who achieved clinical remission at week 8; B: Cumulative remission rates according to previous history of anti-tumor necrosis factor α agent failure (naive vs 1 failure vs ≥ 2 failure) in patients who achieved clinical remission at week 8.
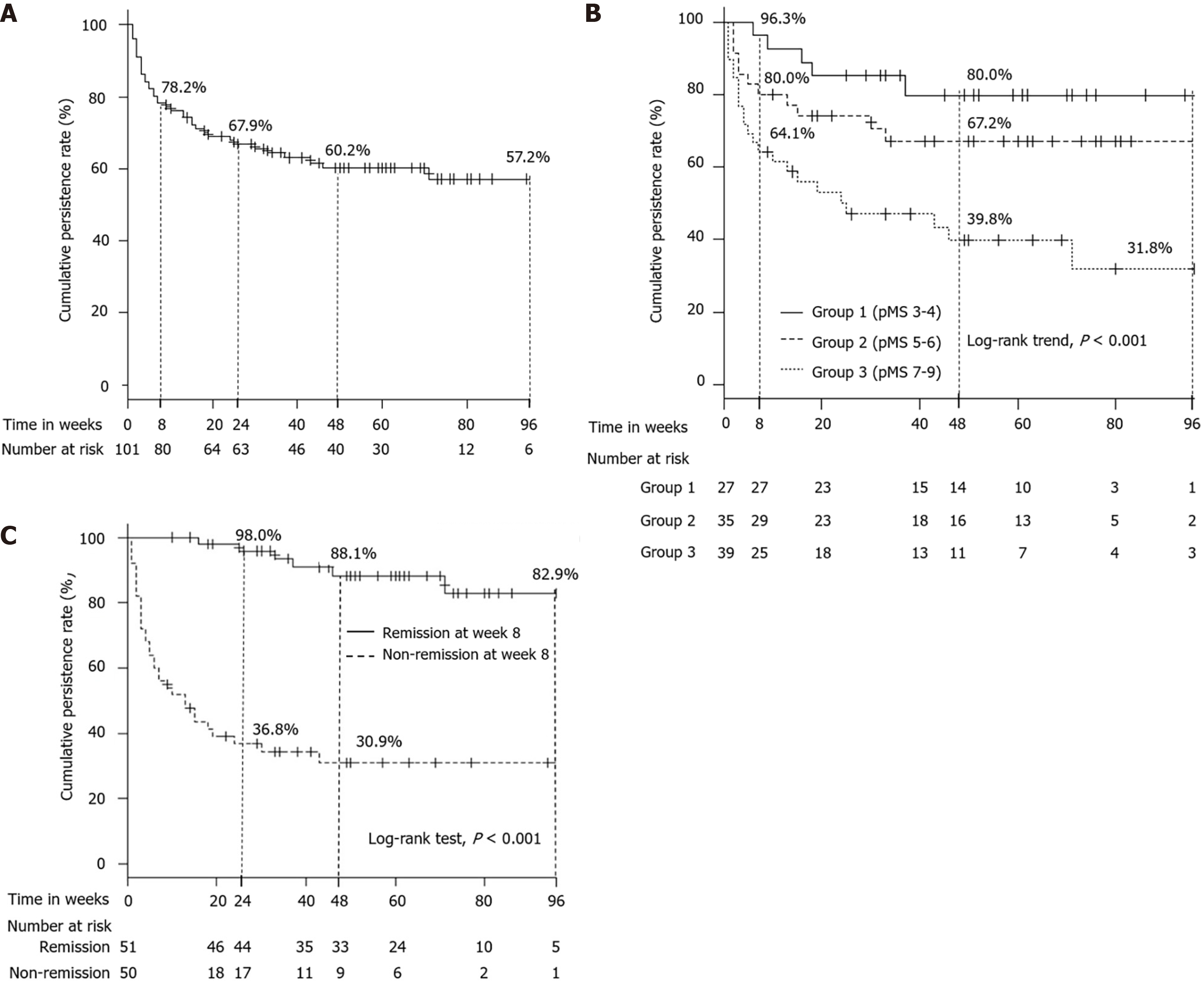
Figure 4 Cumulative persistence rate of tofacitinib administration.
A: Overall cumulative persistence rate of tofacitinib administration; B: Cumulative persistence rate of tofacitinib administration according to partial Mayo Score (pMS) at baseline (Group 1: pMS 3-4, Group 2: pMS 5–6, Group 3: pMS 7–9); C: Cumulative persistence rate of tofacitinib administration between patients who were classified as in clinical remission or non-clinical remission at week 8. pMS: Partial Mayo Score.
Figure 5 Colectomy-free survival after the initiation of tofacitinib.
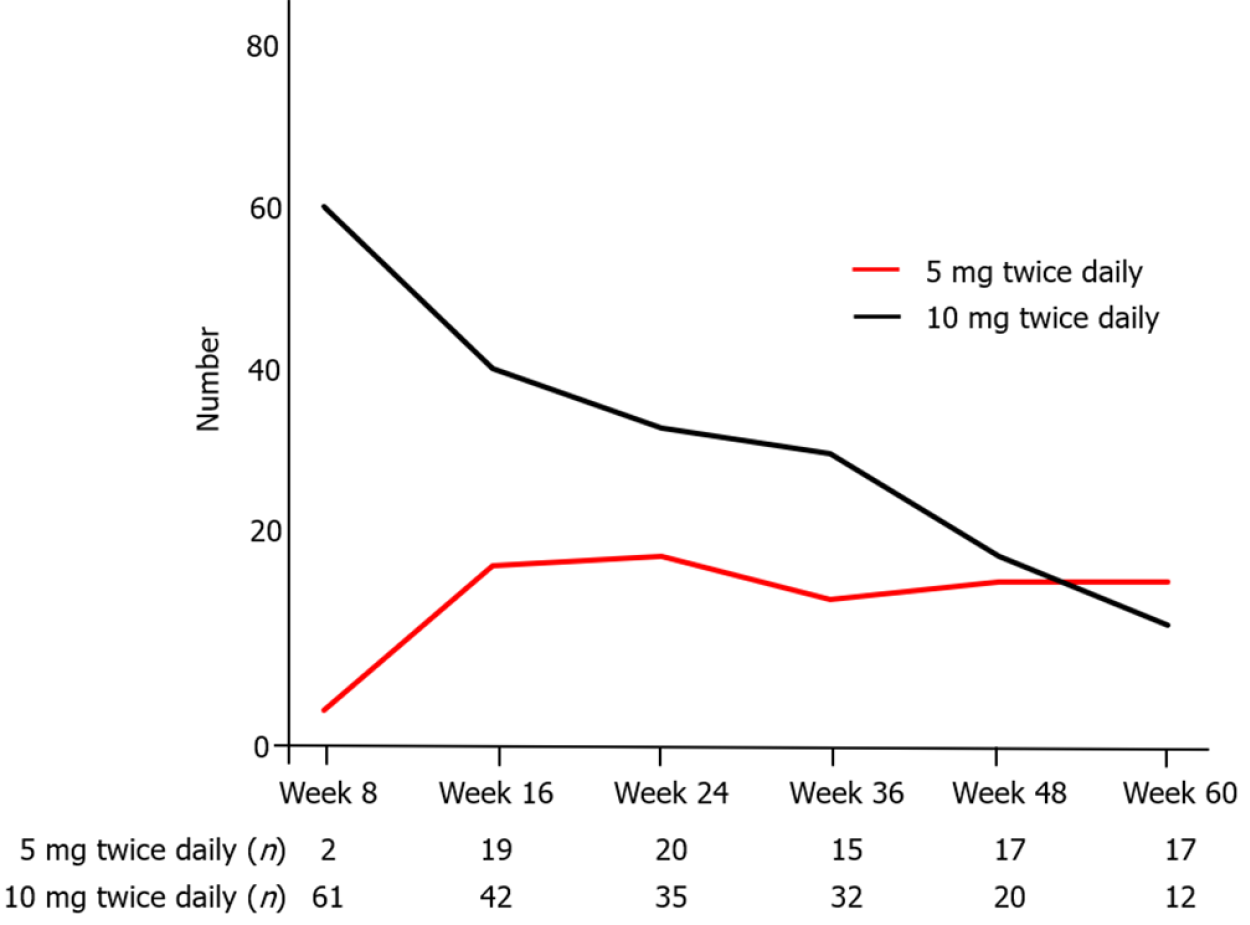
Figure 6 Number of patients in each dosage group of tofacitinib administration at each time point.
Figure 7 Relapse rate and Clinical efficacy for re-increasing the dosage of tofacitinib.
A: Relapse rate after tapering of tofacitinib; B: Clinical efficacy for re-increasing the dosage of tofacitinib due to relapse after tapering of tofacitinib at weeks 4 and 12.
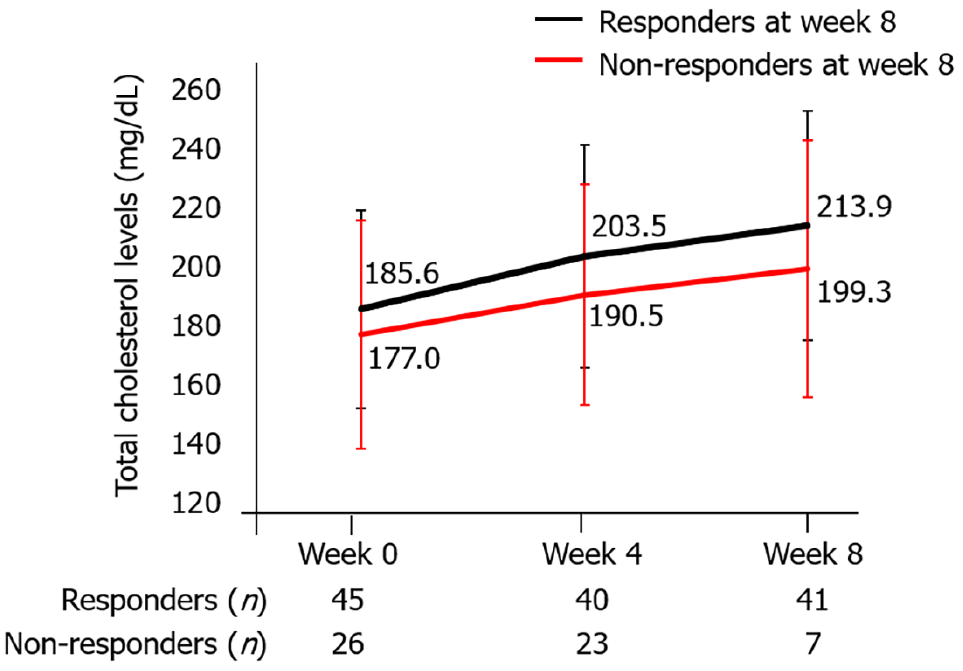
Figure 8 Total cholesterol levels from baseline to week 8 between responders and non-responders at week 8.
Data represent the mean and standard deviation.
Figure 9 Comparison of patient characteristics with and without herpes zoster.
A: Comparison of patient age at tofacitinib initiation between those who developed herpes zoster and those who did not; B: Comparison of the lymphocyte cell count at tofacitinib initiation between patients who developed herpes zoster and those who did not. HZ: Herpes zoster.
- Citation: Kojima K, Watanabe K, Kawai M, Yagi S, Kaku K, Ikenouchi M, Sato T, Kamikozuru K, Yokoyama Y, Takagawa T, Shimizu M, Shinzaki S. Real-world efficacy and safety of tofacitinib treatment in Asian patients with ulcerative colitis. World J Gastroenterol 2024; 30(13): 1871-1886
- URL: https://www.wjgnet.com/1007-9327/full/v30/i13/1871.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i13.1871