Copyright
©The Author(s) 2024.
World J Gastroenterol. Jan 7, 2024; 30(1): 34-49
Published online Jan 7, 2024. doi: 10.3748/wjg.v30.i1.34
Published online Jan 7, 2024. doi: 10.3748/wjg.v30.i1.34
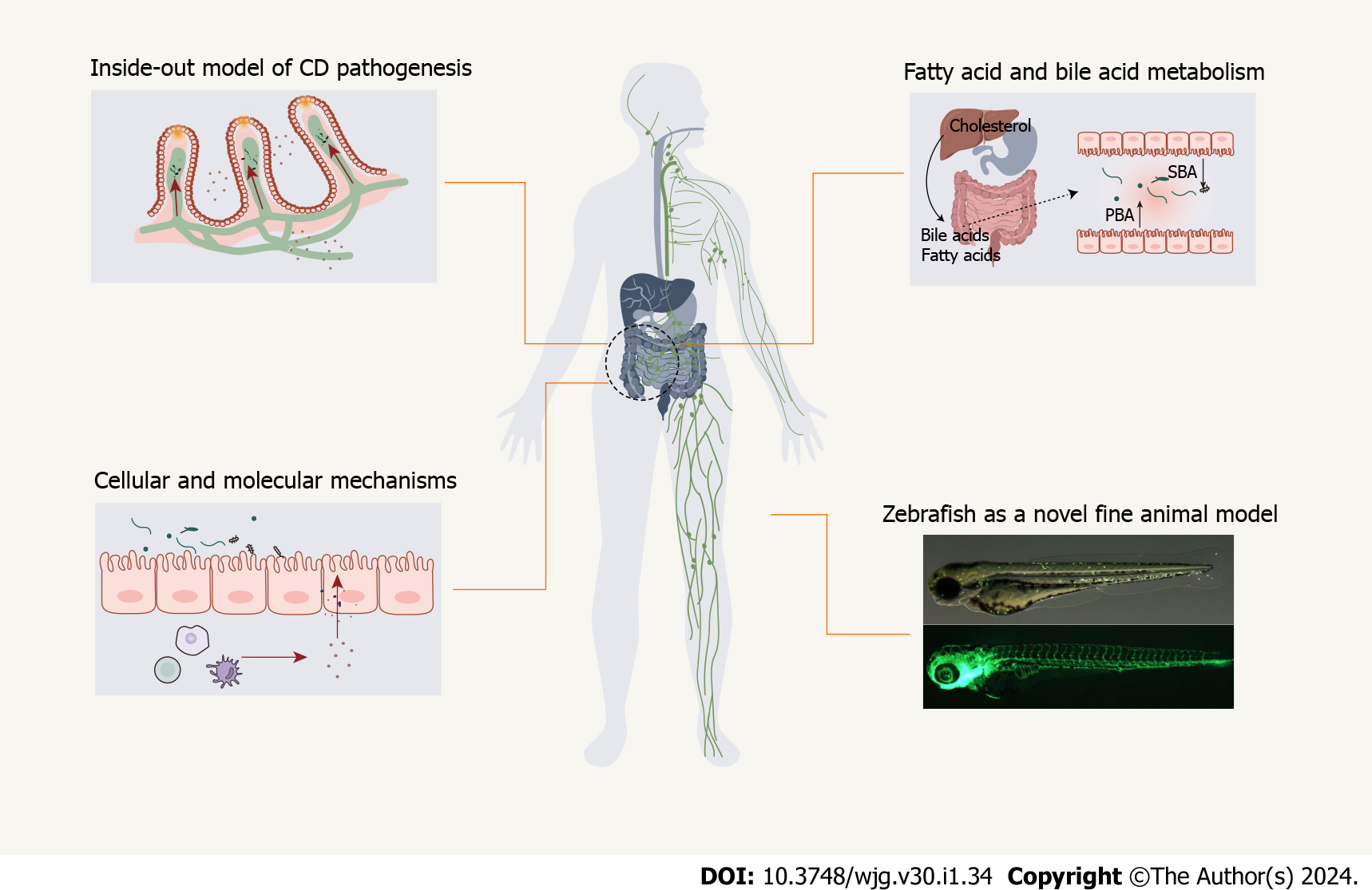
Figure 1 Flowchart for Crohn’s disease as the intestinal manifestation of pan-lymphatic dysfunction.
The following factors are considered: inside-out model, fatty acid/bile acid metabolism, cellular/molecular mechanisms, and zebrafish as a novel fine animal model. CD: Crohn’s disease; PBA: Primary bile acid; SBA: Second bile acid.
Figure 2 Inside-out model of Crohn’s disease pathogenesis.
A: Phase I, intracellular bacteria infect into lymphatic system with few signs of intestinal mucosal injury; B: Phase II, Pathogens infect and persist in intestinal lymphatic tissues, which causes an ‘immunological scar’ in the intestinal lymphatic system. The impaired transport function, lymphangitis, lymphadenopathy, loss of button-like junctions, bacteria translocation to mesenteric lymph nodes and Peyer’s patches, and mesenteric adipose tissue formation have been found in the pathogenesis of Crohn’s disease (CD); C: Phase III, Mucosal injury as the terminal event of CD. Lymphatic dysfunction, including impaired clearance, defective dendritic cells migration, and obstruction, provides opportunities for bacteria and their by-products flowing back to the draining lymphatic vessels and causing mucosal lesions. DC: Dendritic cells; MLN: Mesenteric lymph nodes; PPs: Peyer’s patches.
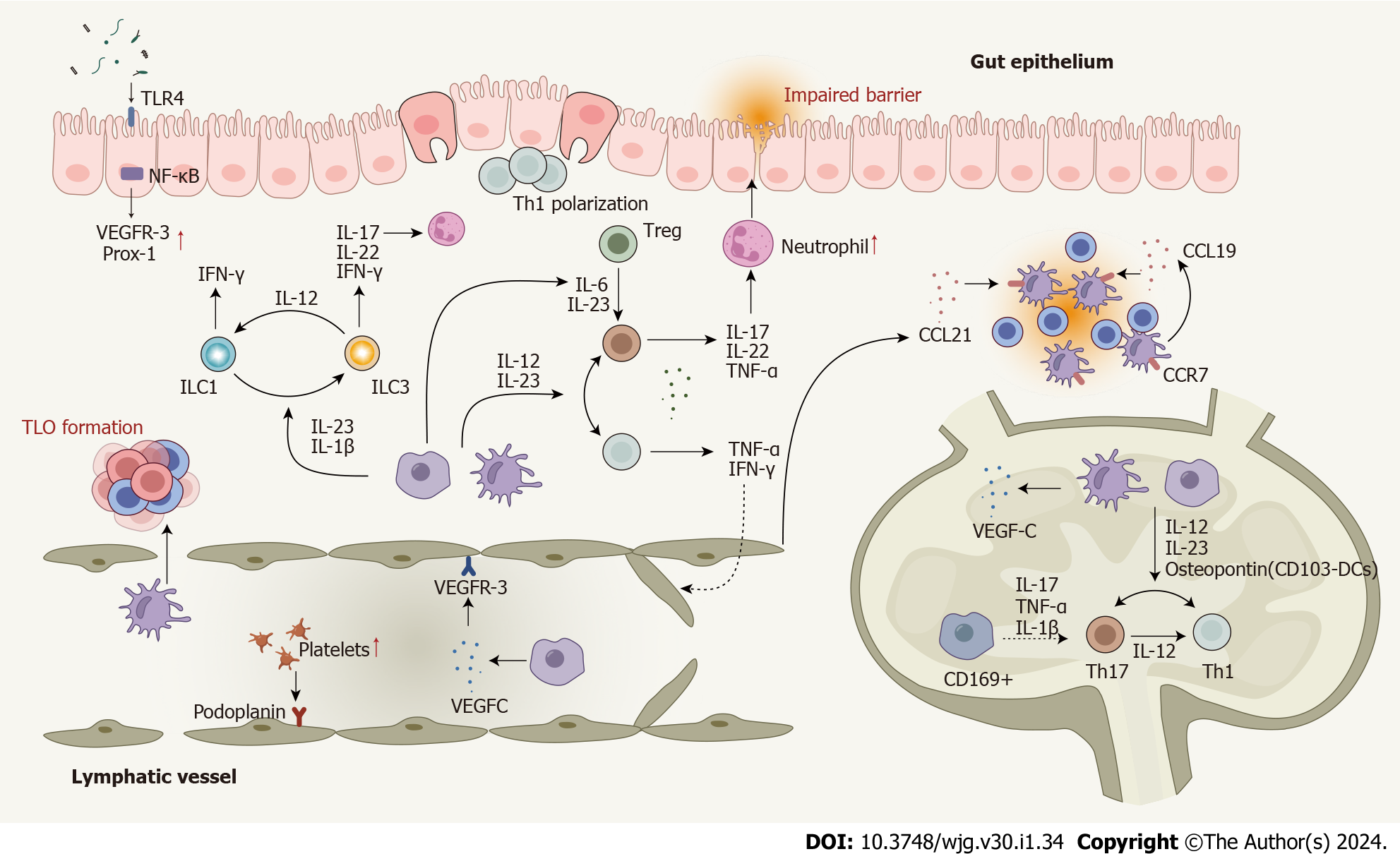
Figure 3 Dysregulated cells, cytokines, and enhanced cell plasticity in the lymphatic system in Crohn’s disease.
T cells are polarized to Th1 and Th17 cells in response to pro-inflammatory cytokines produced by macrophages and dendritic cells (DCs). Several cytokines play roles in Th17-Th1 and Treg-Th17 trans-differentiation. DCs, macrophages, T cells, and B cells contribute to lymphangiogenesis by mediating the expression of lymphangiogenic factors. Impaired lymphatic function and dysregulated chemokines lead mature DCs and T cells away from their usual mesenteric lymph nodes. Innate lymphoid cells (ILCs) also contribute to Crohn’s disease (CD) pathogenesis and enhanced plasticity between ILC1 and ILC3 has been observed. Several signaling pathways involved in the lymphatic system actively participate in the CD pathogenesis. Nuclear factor-kappa B is involved in lymphatic remodeling by directly up-regulating the expression of VEGFR-3 and Prox-1. The mTOR signaling affects leukocyte trafficking through the lymphatic barrier in CD. CD: Crohn’s disease; DC: Dendritic cells; IFN: Interferon; IL: Interleukin; ILC: Innate lymphoid cell; NF-κB: Nuclear factor-kappa B; Prox-1: Prospero homeobox protein 1; Th: T helper; TLO: Tertiary lymphoid organ; TLR4: Toll-like receptor 4; TNF: Tumor necrosis factor; Treg: Regulatory T cell; VEGF: Vascular endothelial growth factor; VEGFR: Vascular endothelial growth factor receptor; MAT: Mesenteric adipose tissue.
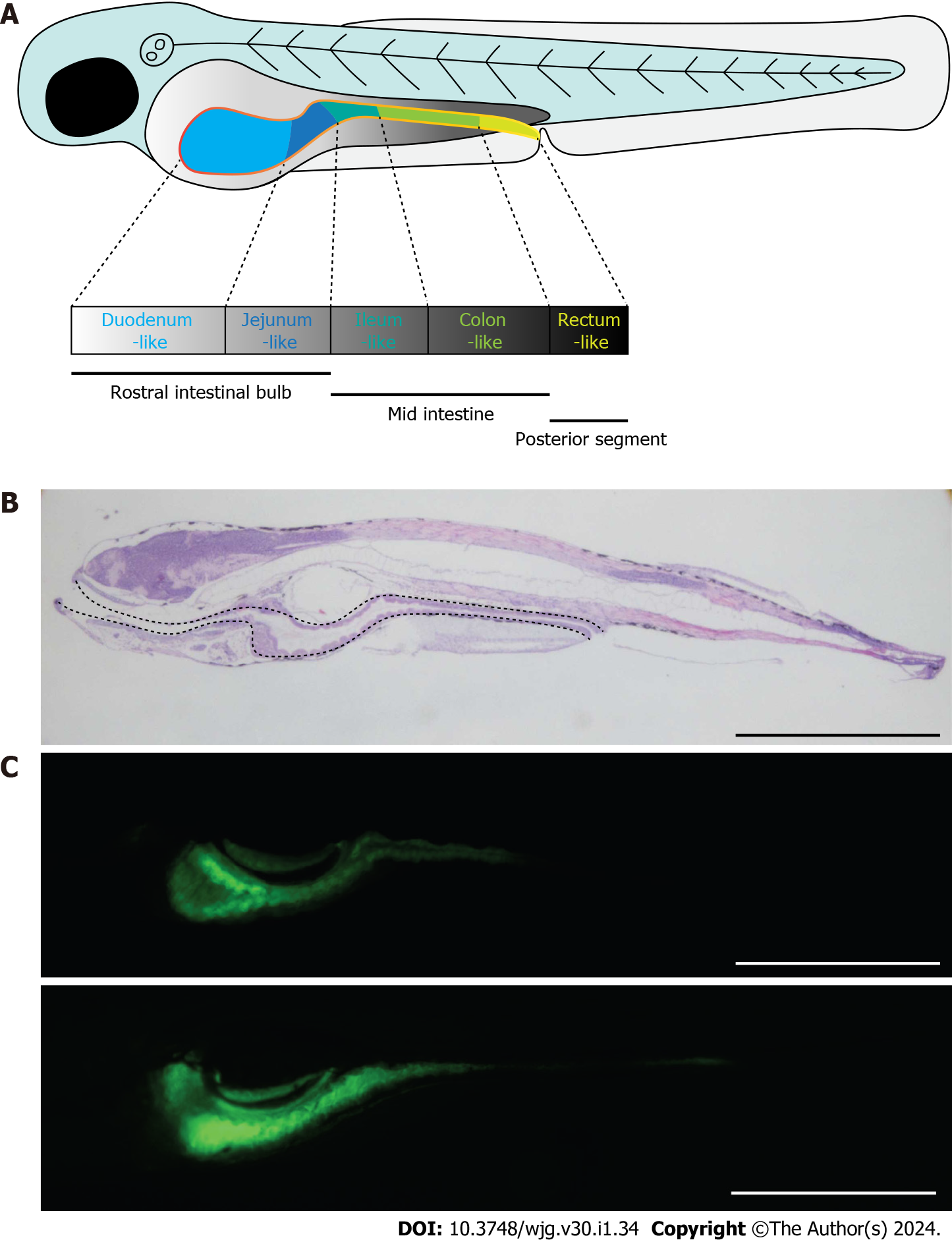
Figure 4 Zebrafish as a good candidate model for the study of Crohn’s disease.
A: Schematic diagram showing the morphology of zebrafish embryo at 5 dpf. The solid yellow line indicates the intestine of the embryo; B: Morphology of zebrafish embryo visualized by hematoxylin and eosin staining of embryonic sections at 5 dpf. Sections were cut along the sagittal plane. The dotted line indicates the whole intestine; C: Fluorescence signals of ET33J1: enhanced green fluorescent protein zebrafish reporter (top) and dichloro-dihydro-fluorescein diacetate treatment (bottom) showing the morphology of the whole intestines at 5 dpf (top) and 7 dpf (bottom). Scale bar: 200 µm. dpf: Days post fertilization.
- Citation: Zhou YW, Ren Y, Lu MM, Xu LL, Cheng WX, Zhang MM, Ding LP, Chen D, Gao JG, Du J, Jin CL, Chen CX, Li YF, Cheng T, Jiang PL, Yang YD, Qian PX, Xu PF, Jin X. Crohn’s disease as the intestinal manifestation of pan-lymphatic dysfunction: An exploratory proposal based on basic and clinical data. World J Gastroenterol 2024; 30(1): 34-49
- URL: https://www.wjgnet.com/1007-9327/full/v30/i1/34.htm
- DOI: https://dx.doi.org/10.3748/wjg.v30.i1.34