Copyright
©The Author(s) 2023.
World J Gastroenterol. Jul 14, 2023; 29(26): 4136-4155
Published online Jul 14, 2023. doi: 10.3748/wjg.v29.i26.4136
Published online Jul 14, 2023. doi: 10.3748/wjg.v29.i26.4136
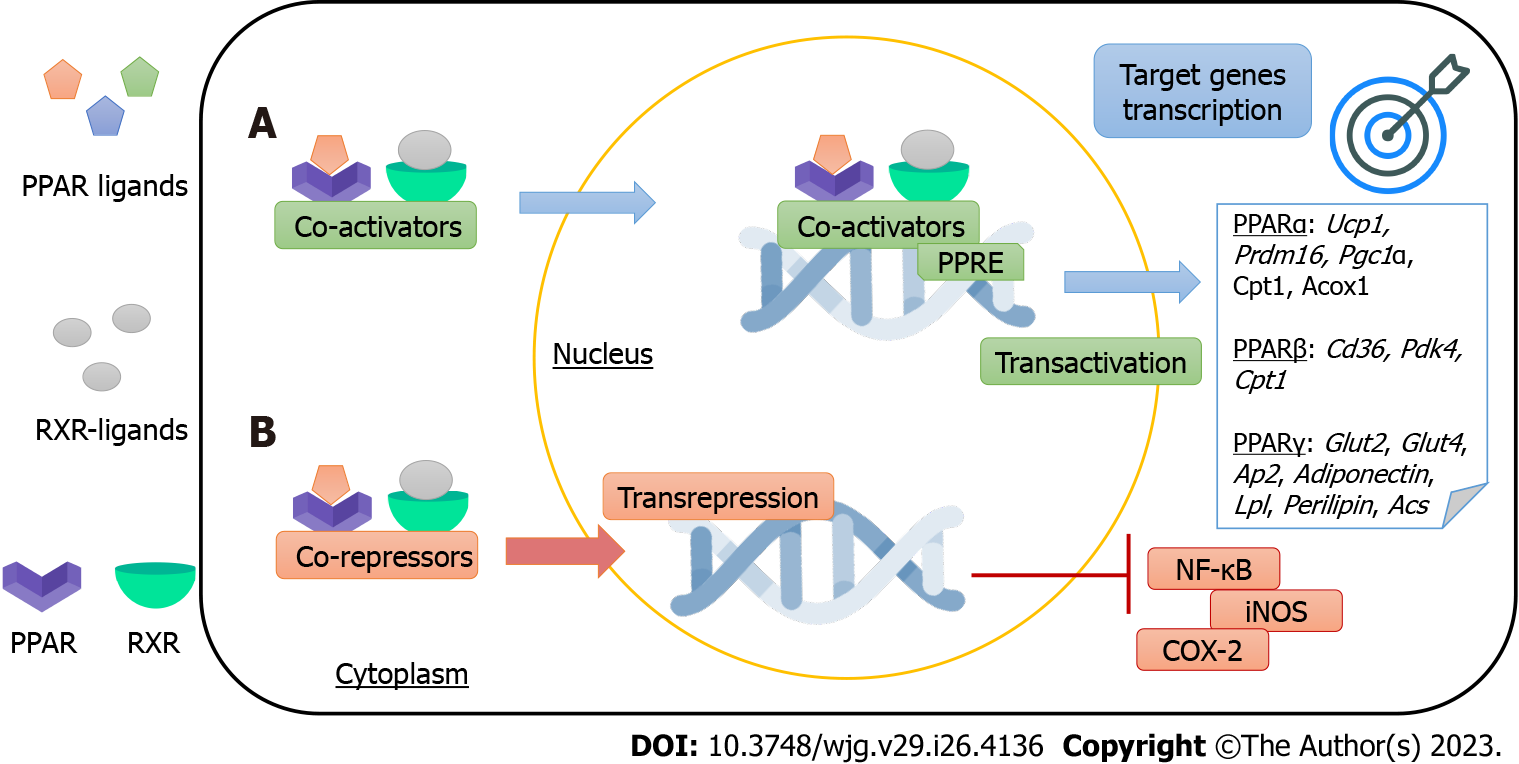
Figure 1 Peroxisome proliferator-activated receptors mechanisms of action.
Proliferator-activated receptors (PPARs) are ligand-activated transcription factors that regulate gene expression through two different mechanisms. A: PPAR-retinoid X receptor heterodimers bind to DNA-specific sequences called peroxisome proliferator-response elements to trigger target genes transcription (transactivation). PPARs’ target genes include thermogenic [Ucp1, PPAR-gamma coactivator 1 alpha (Pgc1α), and PR domain containing 16 (Prdm16)], mitochondrial and peroxisomal [carnitine palmitoyltransferase I (Cpt1), peroxisomal acyl-coenzyme A oxidase (Acox), and pyruvate dehydrogenase kinase 4 (Pdk4)], anti-inflammatory (Adiponectin), insulin-sensitizing [glucose transporter 2 (Glut2), Glut4], and adipocyte metabolism [Cd36, adipocyte protein 2 (Ap2), lipoprotein lipase (Lpl), Perilipin, and acetyl CoA synthetase (Acs)] genes; B: PPARs regulate gene expression through transrepression, a DNA-independent mechanism. For example, PPARs inhibits the activity of nuclear factor kappa b and enzymes, yielding anti-inflammatory effects. Abbreviations: Uncoupling protein 1, Pgc1α, Prdm16, Cpt1, peroxisomal Acox, Pdk4, Glut, Ap2, Lpl, and Acs, inducible nitric oxide synthase, nuclear factor kappa B, and ciclooxigenase-2. PPARs: Peroxisome proliferator-activated receptors; RXR: Retinoid X receptor; PPRE: PPAR-responsive elements; NF-κB: Nuclear factor kappa B; iNOS: Inducible nitric oxide synthase; COX-2: Ciclooxigenase-2.
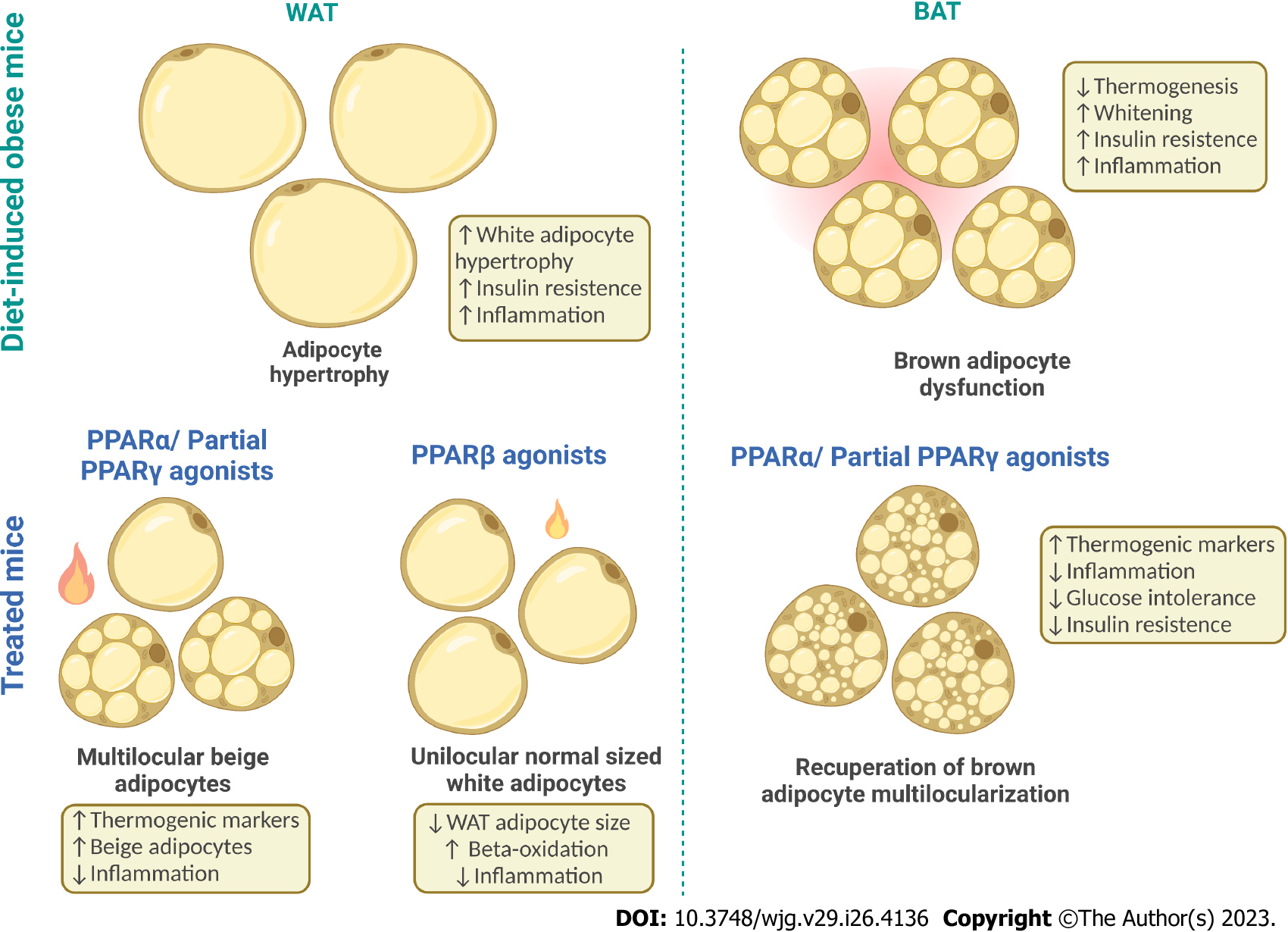
Figure 2 Adipose tissue plasticity and peroxisome proliferator-activated receptor effects.
Under an obesogenic diet, the white adipocytes undergo hypertrophy and profound change in adipokine profile release, leading to growing insulin resistance and low-grade inflammation. Conversely, under adequate stimuli [peroxisome proliferator-activated receptors α (PPARα) and dual PPARα/γ], the subcutaneous white adipose tissue can also undergo browning and form beige adipocytes, an intermediate between white and brown adipocytes with lower thermogenic capacity than brown adipocytes but whose presence points to metabolic homeostasis. Finally, the brown adipose tissue, which has the most potent thermogenic capacity, can express a whitened phenotype when exposed to a lipotoxic milieu. Whitening entails decreased vascularization and pro-inflammatory signals characterizing brown adipocyte dysfunction. In contrast, PPARα and dual PPARα/γ treatments counter whitening, increasing thermogenesis through anti-inflammatory and proangiogenic effects. Made with Biorender (www.biorender.com). PPARs: Peroxisome proliferator-activated receptors; WAT: White adipose tissue; BAT: Brown adipose tissue. Created by BioRender.
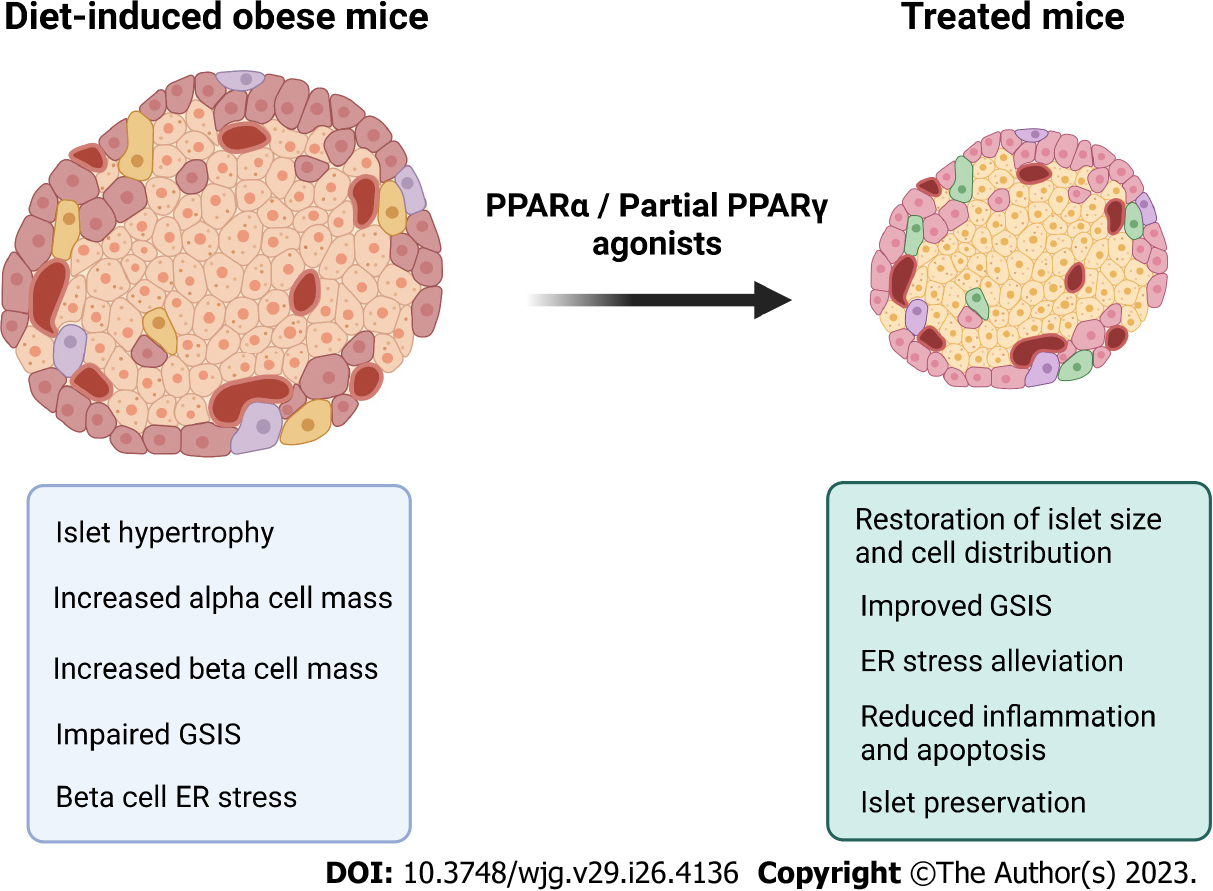
Figure 3 Beneficial effects of Peroxisome proliferator-activated receptor agonist on islet remodelling.
Diet-induced obese mice exhibit enlarged pancreatic islets, with are prone to exhaustion in the long run due to glucolipotoxicity. Peroxisome proliferator-activated receptor α (PPARα) and partial PPARγ activation are promising targets to counter glucolipotoxicity, yielding islet preservation. Made with Biorender (www.biorender.com). PPARs: Peroxisome proliferator-activated receptors; GSIS: Glucose-stimulated insulin secretion; ER: Endoplasmic reticulum. Created by BioRender.
Figure 4 Schematic illustrates hepatic lipotoxicity caused by obesity.
There is an increase in lipolysis, which triggers the intracellular accumulation of fatty acids (FAs) within hepatocytes, resulting in a delay in mitochondrial beta-oxidation followed by an increase in lipogenesis, favoring the deposition of lipid droplets in the hepatic parenchyma, culminating in hepatic steatosis. Concomitantly, there is a reduction in insulin signaling, potentiating inflammation, and endoplasmic reticulum (ER) stress. ER stress is detected by the unfolded protein response-IRE1, PERK, and activating transcription factor 6 (ATF6) in the ER membrane. If it is impossible to reverse the unfolded proteins, ATF4 promotes transcription of the C/element binding protein homologous protein, a transcription factor that induces apoptosis. Made with Biorender (www.biorender.com). TAG: Triacylglycerol; UPR: Unfolded protein response; UPR: Unfolded protein response; PERK: Protein kinase RNA-like ER kinase; ATF: Activating transcription factor 6; IRE6: CHOP: C/EBP homologous protein; NEFAs: Non-esterified fatty acids. Created by BioRender.
Figure 5 Beneficial effects of Peroxisome proliferator-activated receptor activation on the liver.
Peroxisome proliferator-activated receptor α (PPARα) activation mitigates hepatic steatosis through the activation of genes related to mitochondrial biogenesis and beta-oxidation, such as Pgc1α and Cpt1a. Dual PPARα/γ agonists emerge as promising candidates to treat nonalcoholic steatohepatitis once this approach reduces hepatic stellate cells activation, keeping their quiescent stage and impeding fibrogenesis. PPARβ/δ/δ activation entails antisteatotic effects through alleviating the hepatic endoplasmic reticulum stress, inflammation, and apoptosis. Made with Biorender (www.biorender.com). PPAR: Peroxisome proliferator-activated receptor; Pgc1α: Peroxisome proliferator-activated receptor gamma coactivator-1 alpha; Cpt1a: Carnitine palmitoyltransferase 1a; HSC: Hepatic stellate cell; ER: Endoplasmic reticulum. Created by BioRender.
- Citation: Souza-Tavares H, Miranda CS, Vasques-Monteiro IML, Sandoval C, Santana-Oliveira DA, Silva-Veiga FM, Fernandes-da-Silva A, Souza-Mello V. Peroxisome proliferator-activated receptors as targets to treat metabolic diseases: Focus on the adipose tissue, liver, and pancreas. World J Gastroenterol 2023; 29(26): 4136-4155
- URL: https://www.wjgnet.com/1007-9327/full/v29/i26/4136.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i26.4136