Copyright
©The Author(s) 2023.
World J Gastroenterol. Jul 14, 2023; 29(26): 4120-4135
Published online Jul 14, 2023. doi: 10.3748/wjg.v29.i26.4120
Published online Jul 14, 2023. doi: 10.3748/wjg.v29.i26.4120
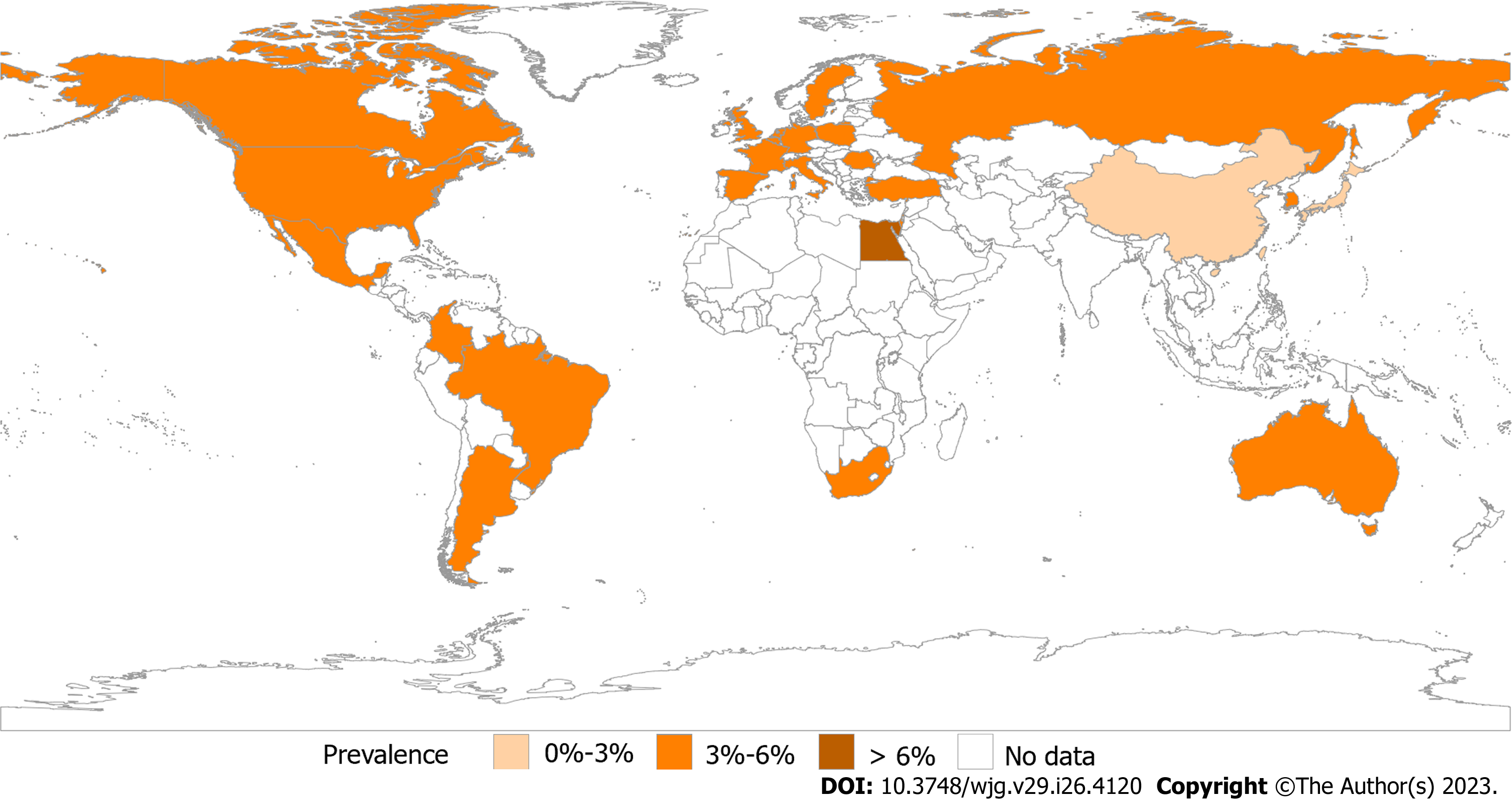
Figure 1 Prevalence of irritable bowel syndrome by Rome IV.
Prevalence of an Internet survey conducted by the Rome Foundation in multiple centers worldwide based on Rome IV. Asia, 1.3%-4.7%; Europe, 3.5%-5.9%; America, 3.5%-5.3%; Australia, 3.5%; Egypt, 7.6%; and South Africa, 5.9%.
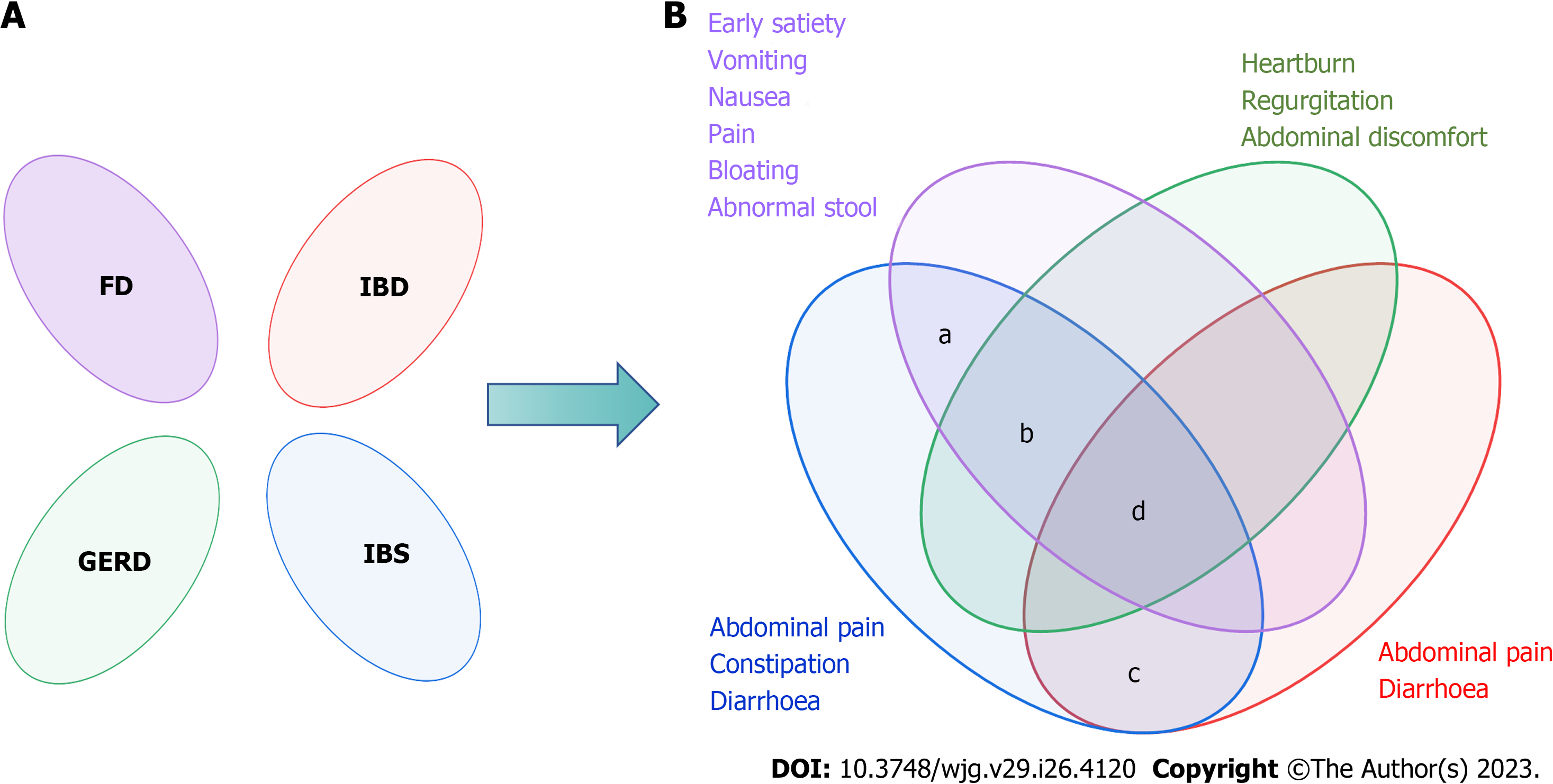
Figure 2 The Rome Foundation's views on the overlap of functional gastrointestinal diseases.
A: Rome I considered the functional bowel disorders to be independent diseases; B: Rome II and Rome III recognized there was overlap between functional gastrointestinal diseases. a: The prevalence of overlap between irritable bowel syndrome (IBS) and functional dyspepsia was 55.3%; b: The overlap between IBS and gastroesophageal reflux disease ranged from 3%-79% in the questionnaire and 10%-74% when diagnosed by endoscopy; c: A 2020 meta-analysis showed that the pooled prevalence of IBS-type symptoms was 32.5%; d: Only 2.3% had esophageal, gastroduodenal, bowel, and anorectal overlap. FD: Functional dyspepsia; IBD: Inflammatory bowel diseases; GERD: Gastroesophageal reflux disease; IBS: Irritable bowel syndrome.
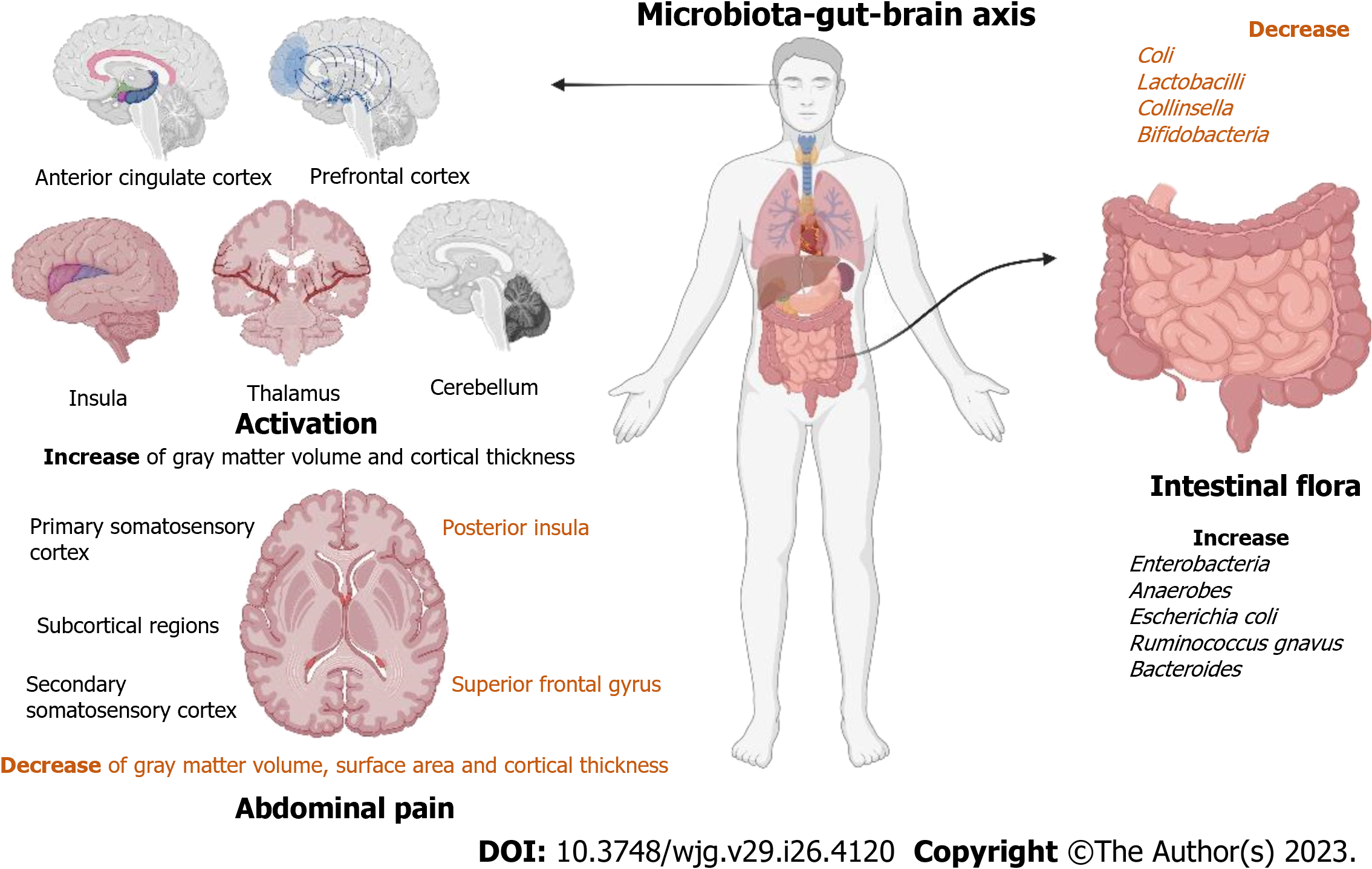
Figure 3 Research progresses on the mechanism of action of the microbiota-gut-brain axis.
Brain changes in patients with irritable bowel syndrome (IBS) are associated with abdominal pain. They have higher activation of the anterior cingulate cortex, prefrontal cortex, insula, thalamus, and cerebellum. They also mainly showed an increase of gray matter volume and cortical thickness in the primary somatosensory cortex, secondary somatic sensory cortex, and subcortical regions and a decrease of gray matter volume, surface area, and cortical thickness in the posterior insula and superior frontal gyrus. Specific changes in the intestinal flora of patients with IBS. The number of Coli, Lactobacilli, Collinsella, and Bifidobacteria in IBS patients decreased, while the number of Enterobacteria, Anaerobes, Escherichia coli, Ruminococcus gnavus, and Bacteroides increased.
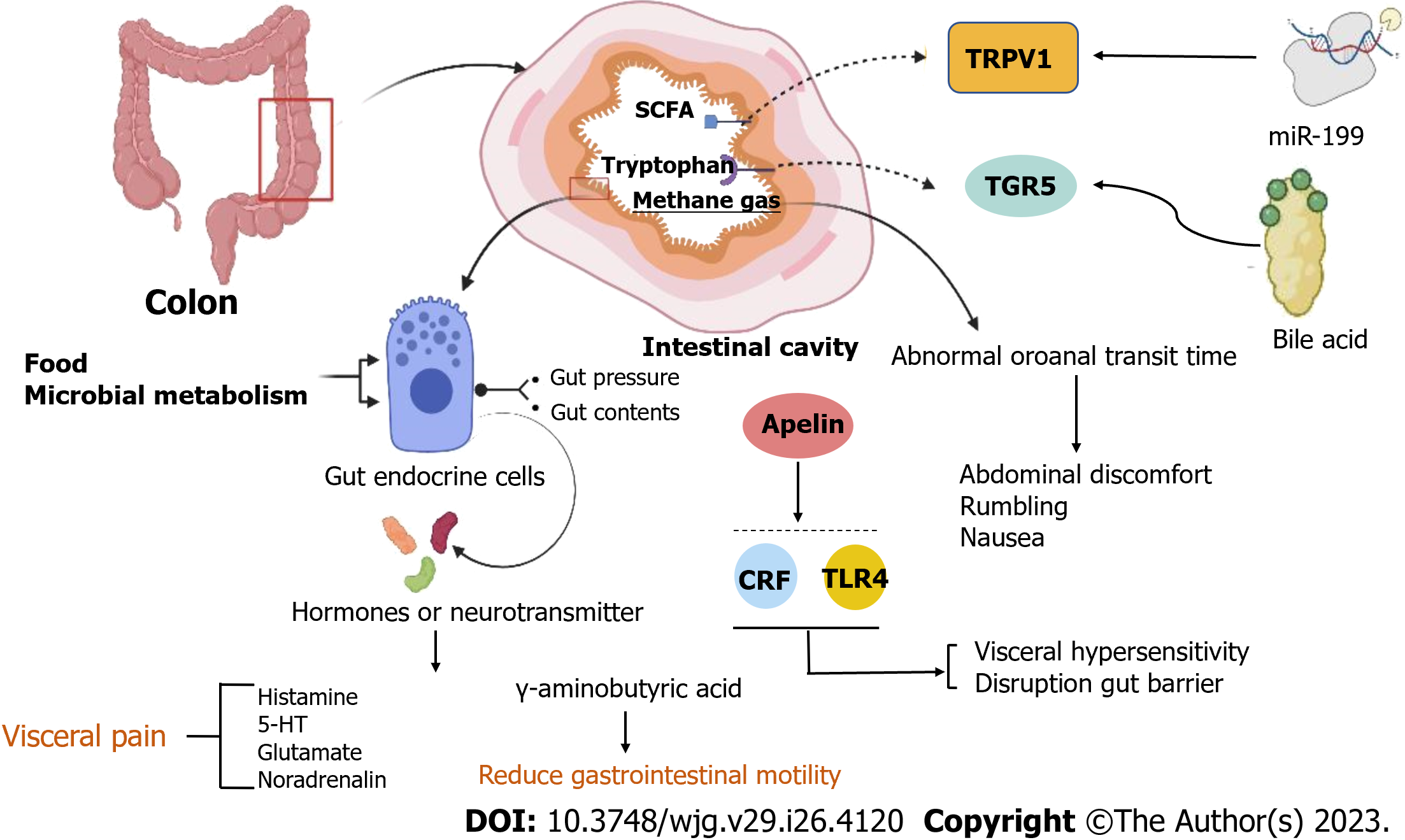
Figure 4 Pathology of irritable bowel syndrome in the intestinal.
Food and microbial metabolism stimulate the gut's endocrine cells to release hormones and neurotransmitters, leading to visceral pain and reducing gastrointestinal motility. Apelin, corticotropin-releasing factor, and Toll-like receptor 4-proinflammatory cytokine signaling lead to visceral hypersensitivity and disruption of the gut barrier. The concentrations of hydrogen and methane are related to abnormal oroanal transit time (OATT), and a more rapid OATT was associated with a higher severity of abdominal discomfort, rumbling, and nausea. Decreasing miR-199 caused visceral hypersensitivity and augmented visceral pain in patients with irritable bowel syndrome (IBS) through translational upregulation of TRPV1. Colonic mucosal protein expression and faecal bile acids were correlated with the symptom severity of IBS-D patients. CRF: Corticotropin-releasing factor; TLR4: Toll-like receptor 4.
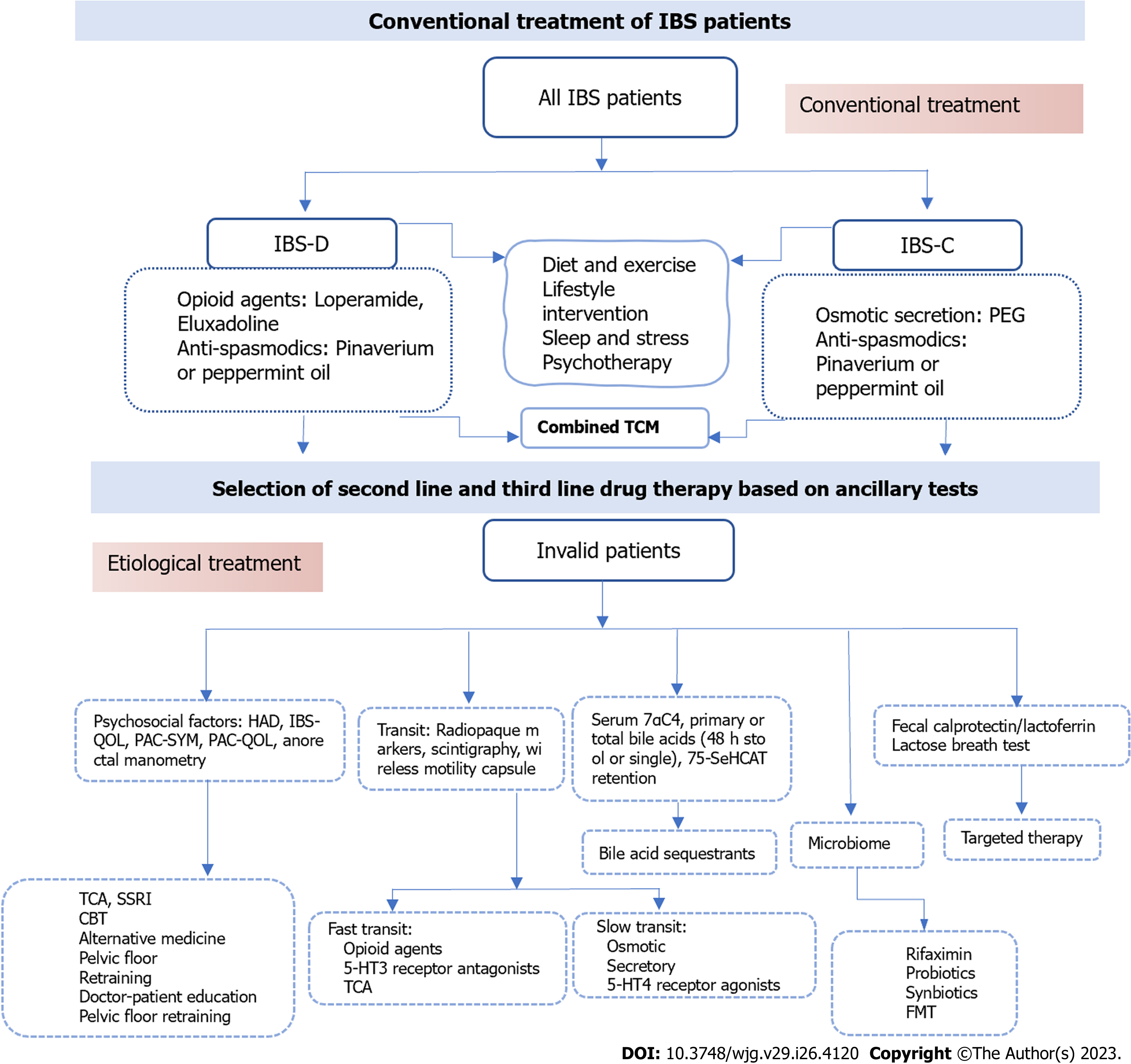
Figure 5 The conventional treatment and further treatment of irritable bowel syndrome patients.
TCM: Traditional Chinese treatment; CBT: Cognitive behavioral treatment; HAD: Hospital Anxiety and Depression scale; IBS-QOL: Irritable Bowel Syndrome-Quality of Life Questionnaire; PAC-QOL: Patient Assessment of Constipation-Quality of Life Questionnaire; PAC-SYM: PAC-Symptoms Questionnaire; 7αC4: 7α-hydroxy-4-cholesten-3-one; 75-SeHCAT: 75-selenium homocholic acid taurine; TCA: Tricyclic Antidepressants; SSRI: Selective Serotonin Reuptake Inhibitors; IBS: Irritable bowel syndrome; IBS-C: IBS-constipation; IBS-D: IBS-diarrhea.
- Citation: Huang KY, Wang FY, Lv M, Ma XX, Tang XD, Lv L. Irritable bowel syndrome: Epidemiology, overlap disorders, pathophysiology and treatment. World J Gastroenterol 2023; 29(26): 4120-4135
- URL: https://www.wjgnet.com/1007-9327/full/v29/i26/4120.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i26.4120