Copyright
©The Author(s) 2023.
World J Gastroenterol. Jul 7, 2023; 29(25): 3999-4008
Published online Jul 7, 2023. doi: 10.3748/wjg.v29.i25.3999
Published online Jul 7, 2023. doi: 10.3748/wjg.v29.i25.3999
Figure 1 Effect of insulin resistance on adipose tissue lipolysis.
Under normal circumstances, insulin inhibits lipolysis in adipose tissue by inducing the hormone sensitive lipase, thus decreasing the release of excessive free fatty acids (FFAs) into the serum. However, in an insulin resistance state, inhibition of lipolysis is blocked, increasing serum FFAs, which eventually increase the influx of lipids into the liver. All the figures were created using BioRender. AT: Adipose tissue; IR: Insulin resistance.
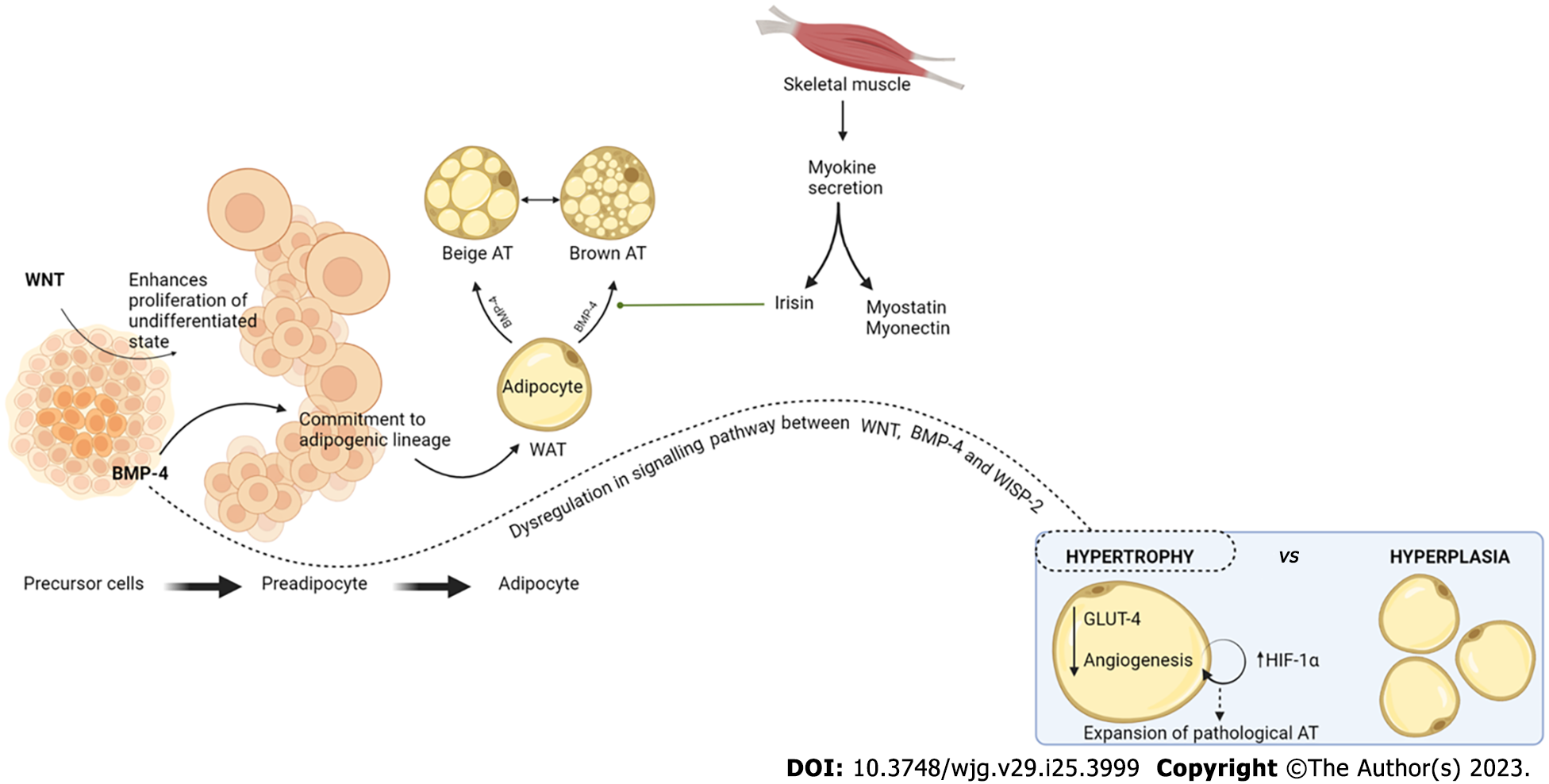
Figure 2 Adipose tissue dysfunction in metabolic dysfunction–associated fatty liver disease.
Adipocyte precursor cells undergo initial proliferation through the wingless-related integration site (WNT) signaling pathway and thereafter commitment to the adipogenic lineage by bone morphogenetic protein 4 (BMP-4) stimulus, until its conversion to preadipocytes and later on to mature adipocytes. White adipose tissue can undergo beiging or browning under the influence of two main stimuli: BMP-4 and irisin. Browning of adipose tissue (AT) implies higher catabolic and oxidation rates, In the case of WNT, BMP-4, and WNT-1 inducible signaling pathway protein 2 pathway dysregulation, there is hypertrophy of AT. Physiologically, hyperplasia through the proliferation process mentioned is the appropriate mechanism for AT expansion. Pathologically, however, hypertrophy of AT leads to decreased levels of intracellular glucose transporter 4 and limited angiogenesis. Limited angiogenesis stimulates the hypoxia inducible factor 1 alpha (HIF-1a), stimulating further AT hypertrophy, creating a vicious cycle in the expansion of pathological AT. WAT: White adipose tissue; WISP-2: WNT-1 inducible signaling pathway protein 2; GLUT-4: Glucose transporter 4.
- Citation: Pal SC, Méndez-Sánchez N. Insulin resistance and adipose tissue interactions as the cornerstone of metabolic (dysfunction)-associated fatty liver disease pathogenesis. World J Gastroenterol 2023; 29(25): 3999-4008
- URL: https://www.wjgnet.com/1007-9327/full/v29/i25/3999.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i25.3999