Copyright
©The Author(s) 2023.
World J Gastroenterol. Jan 14, 2023; 29(2): 332-342
Published online Jan 14, 2023. doi: 10.3748/wjg.v29.i2.332
Published online Jan 14, 2023. doi: 10.3748/wjg.v29.i2.332
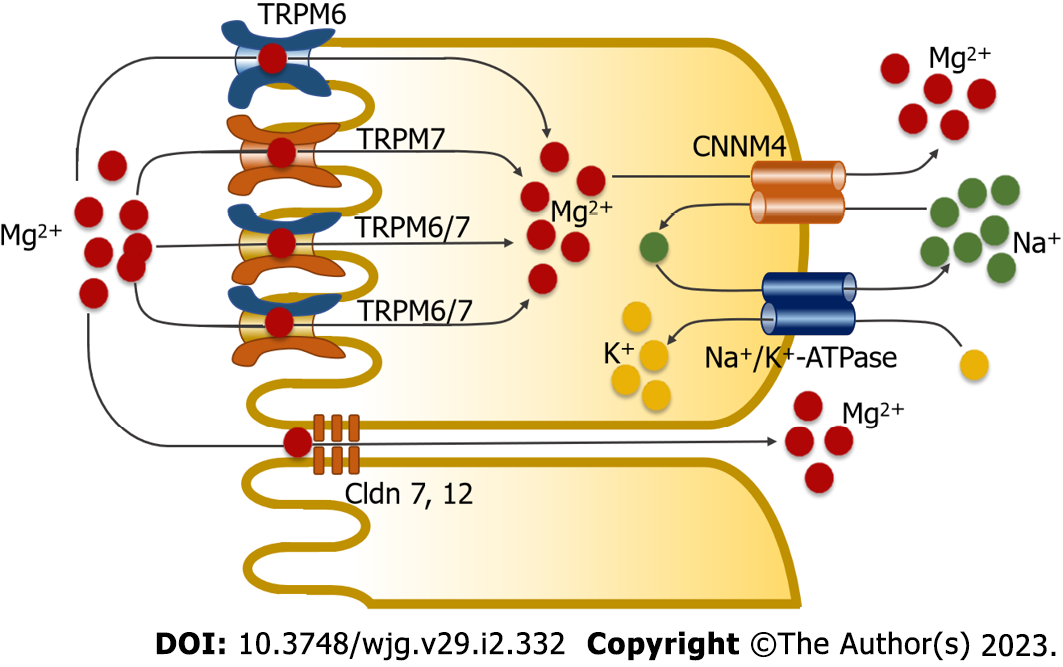
Figure 1 Magnesium absorption in the small intestine through two absorption pathways.
The transcellular transport mechanism involves magnesium (Mg2+) influx into enterocytes through the transient receptor potential melastatin 6 homodimer channel (TRPM6), transient receptor potential melastatin 7 homodimer channel (TRPM7), and transient receptor potential melastatin 6/7 heterodimer channel (TRPM6/7). Cystathionine β-synthase domain divalent metal cation transport mediator 4 (CNNM4) mediates basolateral Mg2+ extrusion by means of secondary active transport. In the paracellular mechanism, Mg2+ moves through tight-associated paracellular pores of Claudin 7 (Cldn 7) and Claudin 12 (Cldn 12).
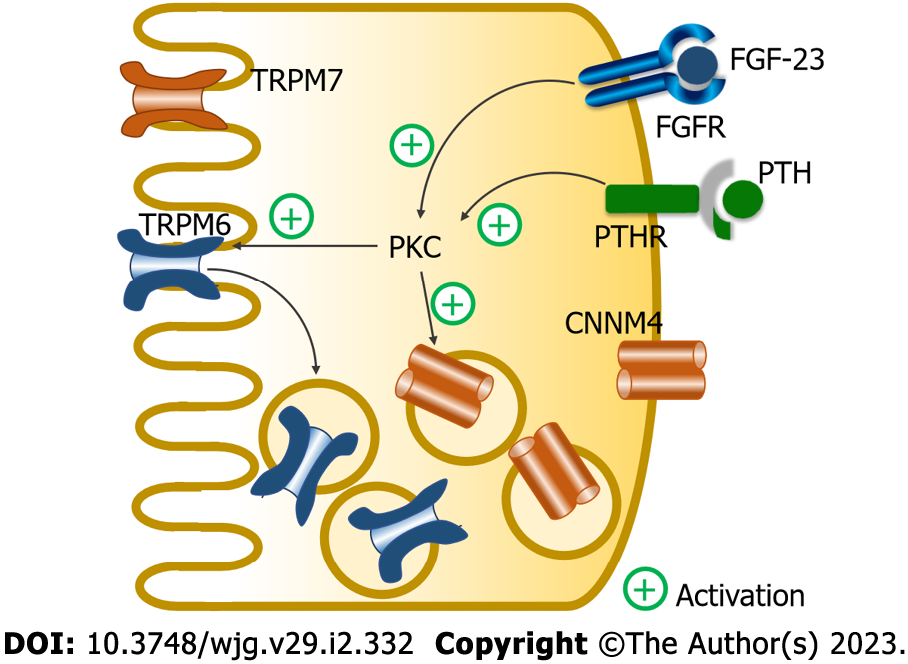
Figure 2 Fibroblast growth factor-23 and parathyroid hormone regulate magnesium absorption in the small intestine.
Fibroblast growth factor-23 (FGF-23) and parathyroid hormone (PTH) act through their corresponding receptors to suppress magnesium absorption in the protein kinase C (PKC)-dependent pathway; they suppressed membrane transient receptor potential melastatin 6 homodimer channel (TRPM6) expression, which leads to the suppression of membrane transient receptor potential melastatin 6/7 (TRPM6/7) expression. FGF-23 and PTH also increase cytosolic cystathionine β-synthase domain divalent metal cation transport mediator 4 (CNNM4) expression. TRPM7: Transient receptor potential melastatin 7 homodimer channel; FGFR: Fibroblast growth factor receptor; PTHR: Parathyroid hormone receptor.
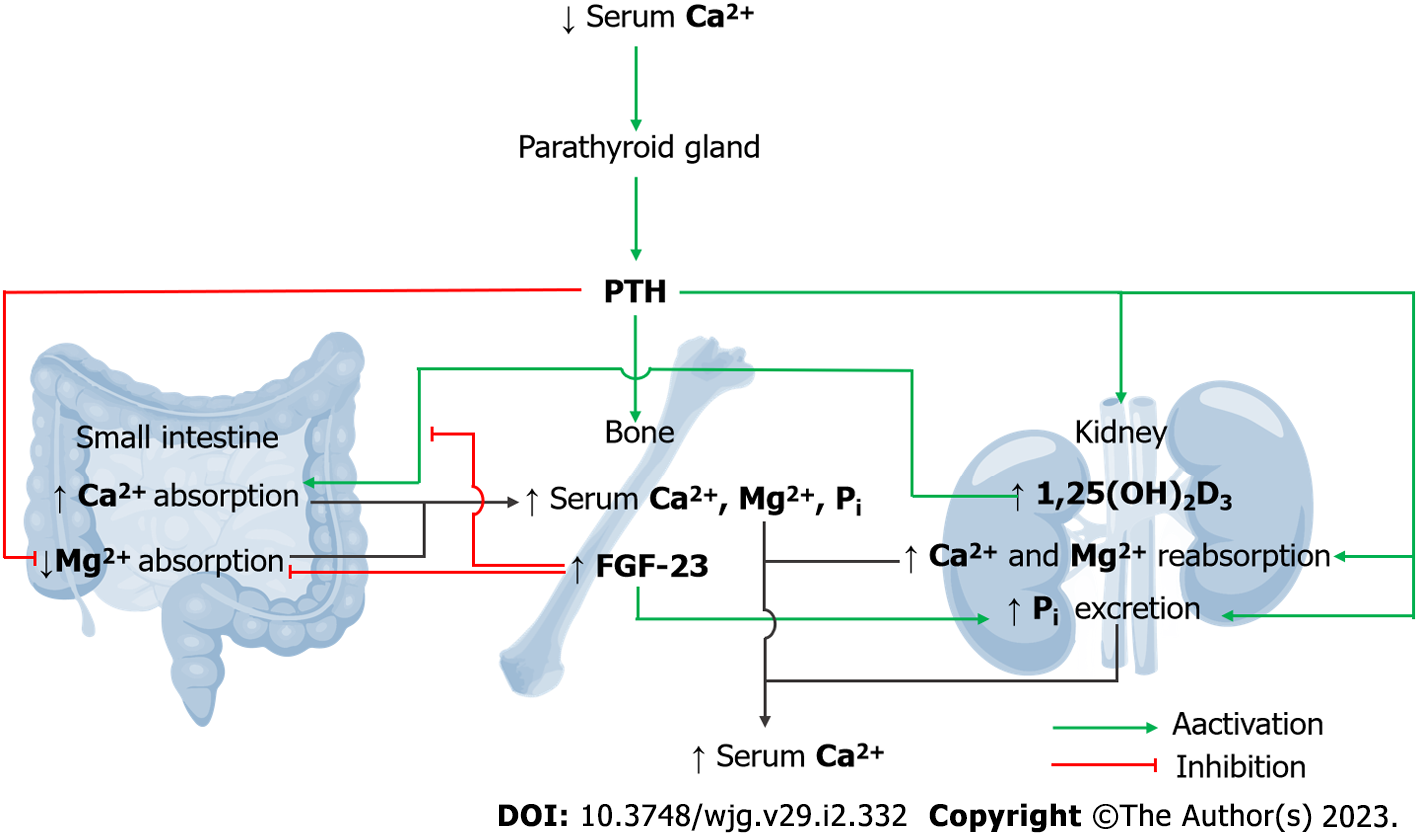
Figure 3 Integrated magnesiotropic action of fibroblast growth factor-23 and parathyroid hormone.
Parathyroid hormone (PTH) stimulates the bone resorption process, which increases plasma calcium (Ca2+), inorganic phosphate (Pi), and magnesium (Mg2+) levels. PTH stimulates the release of 1,25-dihydroxy vitamin D3 (1,25(OH)2D3) to subsequently induce small-intestinal Ca2+ absorption. PTH also activates renal tubular Ca2+ and Mg2+ reabsorption. Plasma Pi and PTH trigger the release of fibroblast growth factor-23 (FGF-23) to abolish 1,25(OH)2D3-induced intestinal Ca2+ absorption. PTH and FGF-23 synergistically suppress small intestinal absorption of dietary Mg2+. PTH and FGF-23 induce urinary Pi excretion.
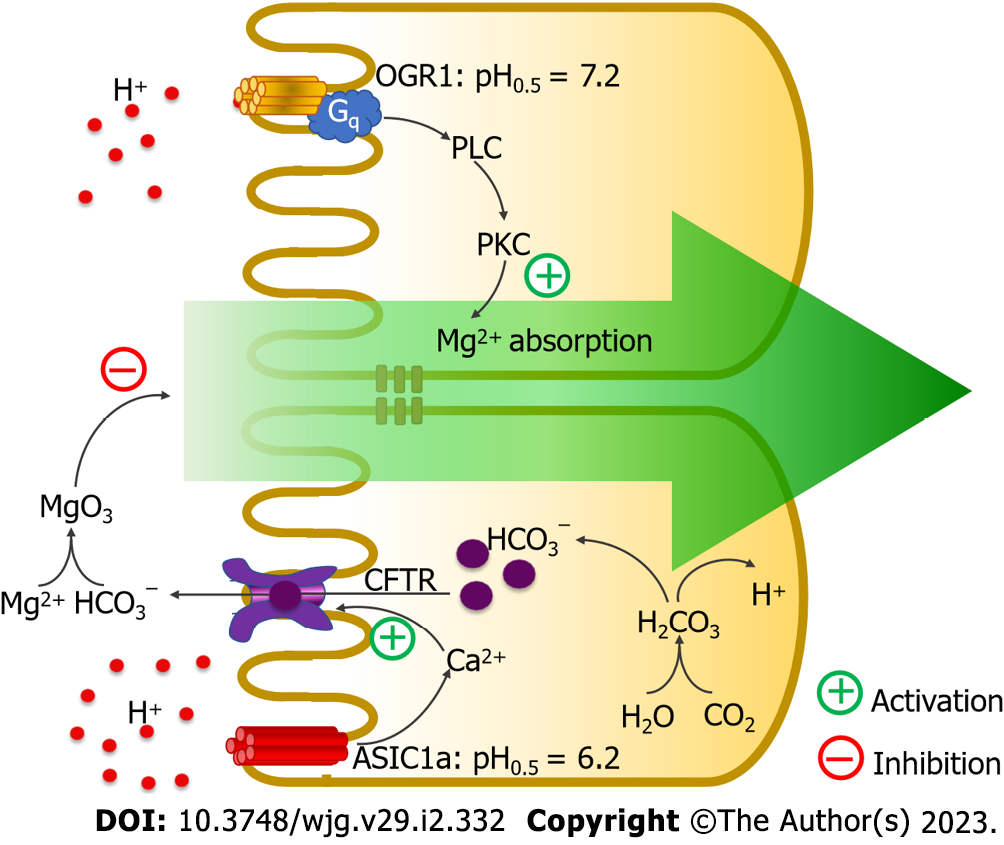
Figure 4 Ovarian cancer G protein-coupled receptor 1 and acid-sensing ion-channel 1a modulate intestinal magnesium absorption.
Activation of ovarian cancer G protein-coupled receptor 1 (OGR1) triggers the phospholipase C (PLC)–protein kinase C (PKC) signaling pathway to activate intestinal magnesium (Mg2+) absorption. Activation of acid-sensing ion-channel 1a (ASIC1a) activates intracellular calcium (Ca2+) signaling to induce mucosal bicarbonate secretion in a cystic fibrosis transmembrane conductance regulator (CFTR)-dependent mechanism. Secreted bicarbonate (HCO3−) reduces free soluble Mg2+ and suppresses intestinal Mg2+ absorption. H: Hydrogen.
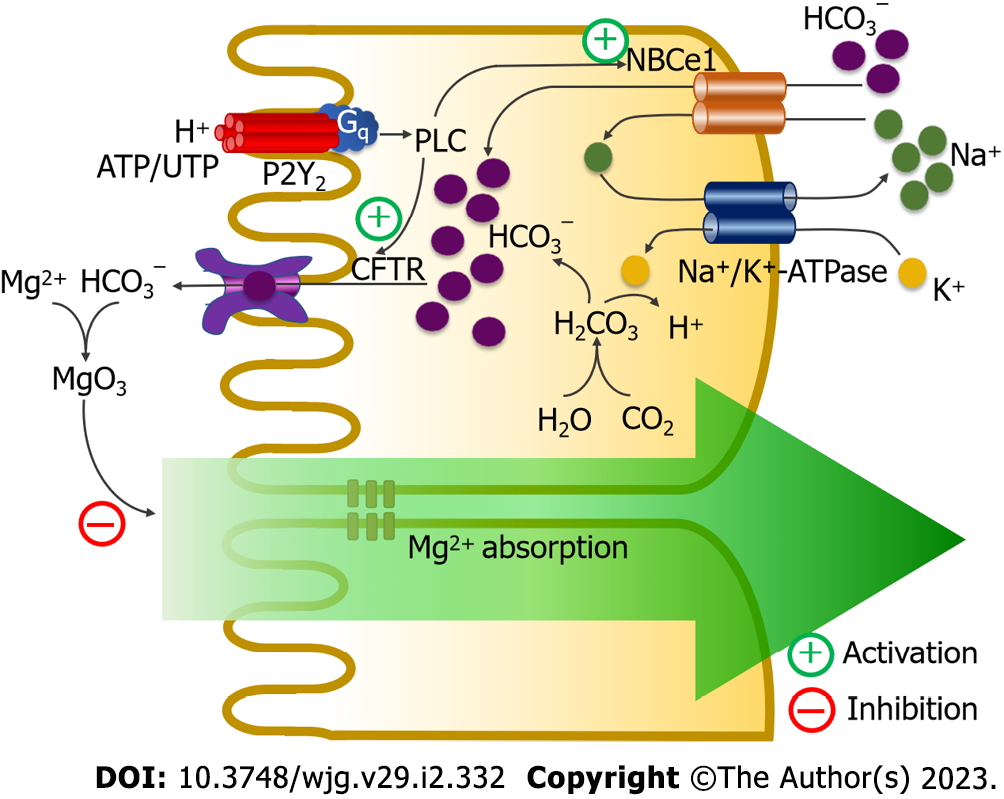
Figure 5 P2Y2 purinoceptors modulate intestinal magnesium absorption.
Activation of P2Y2 purinoceptor stimulates luminal cystic fibrosis transmembrane conductance regulator and basolateral Na+-HCO3− cotransporter-1 (NBCe1) activities through a phospholipase C (PLC)-dependent mechanism. Active cystic fibrosis transmembrane conductance regulator (CFTR) and Na+-HCO3− cotransporter-1 induce mucosal bicarbonate (HCO3−) secretion, which reduces luminal free magnesium (Mg2+) and suppresses Mg2+ absorption. Ca2+: Calcium; H: Hydrogen; K: Potassium; Na+: Sodium.
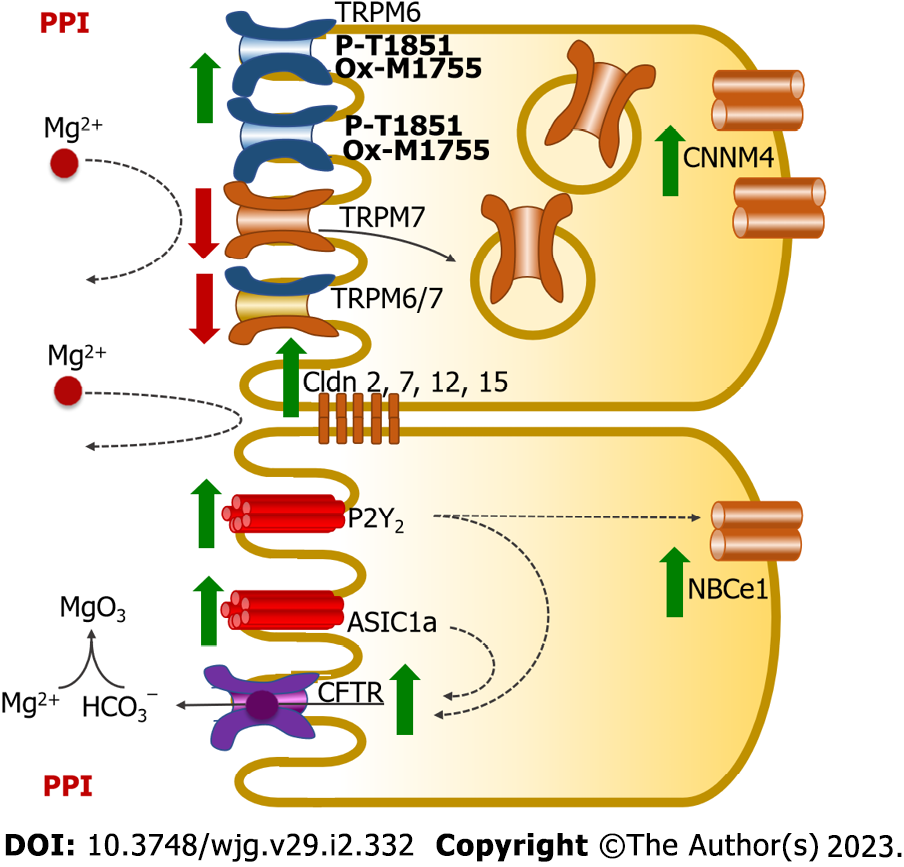
Figure 6 Mechanism of proton pump inhibitor-suppressed small intestinal magnesium absorption in proton pump inhibitor-induced hypomagnesemia rats.
Proton pump inhibitor (PPI) suppresses membrane transient receptor potential melastatin 7 homodimer channel (TRPM7) and transient receptor potential melastatin 6/7 homodimer channel (TRPM6/7) expression but increases membrane transient receptor potential melastatin 6 homodimer channel (TRPM6) and cystathionine β-synthase domain divalent metal cation transport mediator 4 (CNNM4) expression. PPI induces phosphorylation of the T1851 residue and oxidation of the M1755 residue of the membrane TRPM6 channel, which reduces their channel permeability. These PPI effects reduce transcellular magnesium (Mg2+) absorption. Overexpression of claudin 2 (Cldn2), claudin 7 (Cldn7), claudin 12 (Cldn12), and claudin 15 (Cldn15) reduces paracellular permeability, which suppresses paracellular Mg2+ absorption. PPI also enhances P2Y2- and acid-sensing ion-channel 1a (ASIC1a)-suppressed intestinal Mg2+ absorption. CFTR: Cystic fibrosis transmembrane conductance regulator; HCO3−: Bicarbonate; NBCe1: Na+-HCO3− cotransporter-1.
- Citation: Chamniansawat S, Suksridechacin N, Thongon N. Current opinion on the regulation of small intestinal magnesium absorption. World J Gastroenterol 2023; 29(2): 332-342
- URL: https://www.wjgnet.com/1007-9327/full/v29/i2/332.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i2.332