Copyright
©The Author(s) 2023.
World J Gastroenterol. May 14, 2023; 29(18): 2784-2797
Published online May 14, 2023. doi: 10.3748/wjg.v29.i18.2784
Published online May 14, 2023. doi: 10.3748/wjg.v29.i18.2784
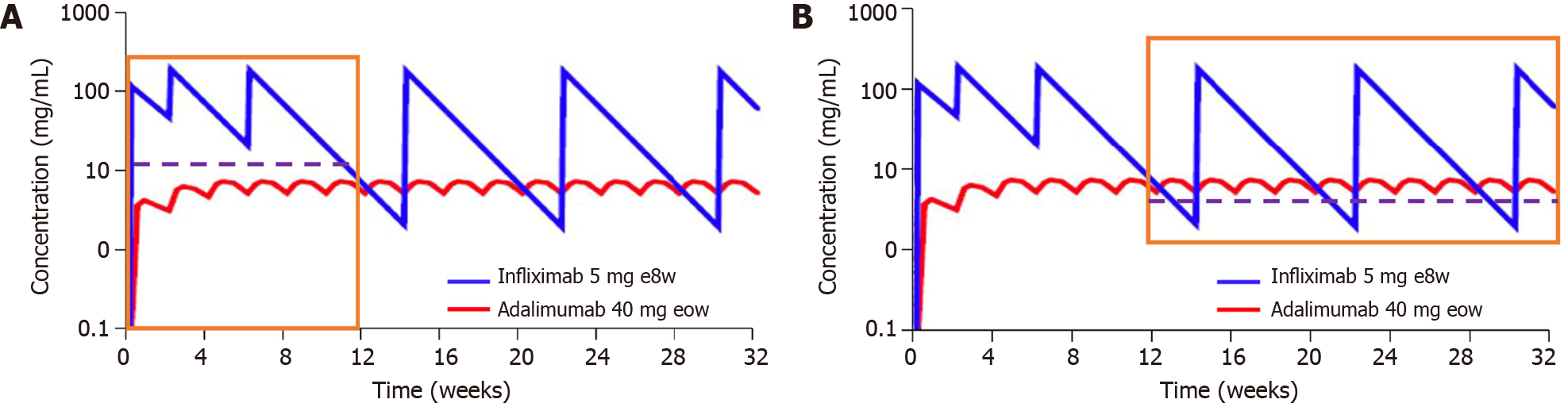
Figure 1 The pharmacokinetic profile of an intravenously or subcutaneously administered anti-tumor necrosis factor agent.
A: According to a theoretical induction dosing regimen; B: According to a theoretical maintenance dosing regimen. TNF: tumor necrosis factor. Citation: Gibson DJ, Ward MG, Rentsch C, Friedman AB, Taylor KM, Sparrow MP, Gibson PR. Review article: determination of the therapeutic range for therapeutic drug monitoring of adalimumab and infliximab in patients with inflammatory bowel disease. Aliment Pharmacol Ther 2020; 51: 612-628. Copyright ©John Wiley & Sons Ltd. 2020. Published by John Wiley & Sons[31].
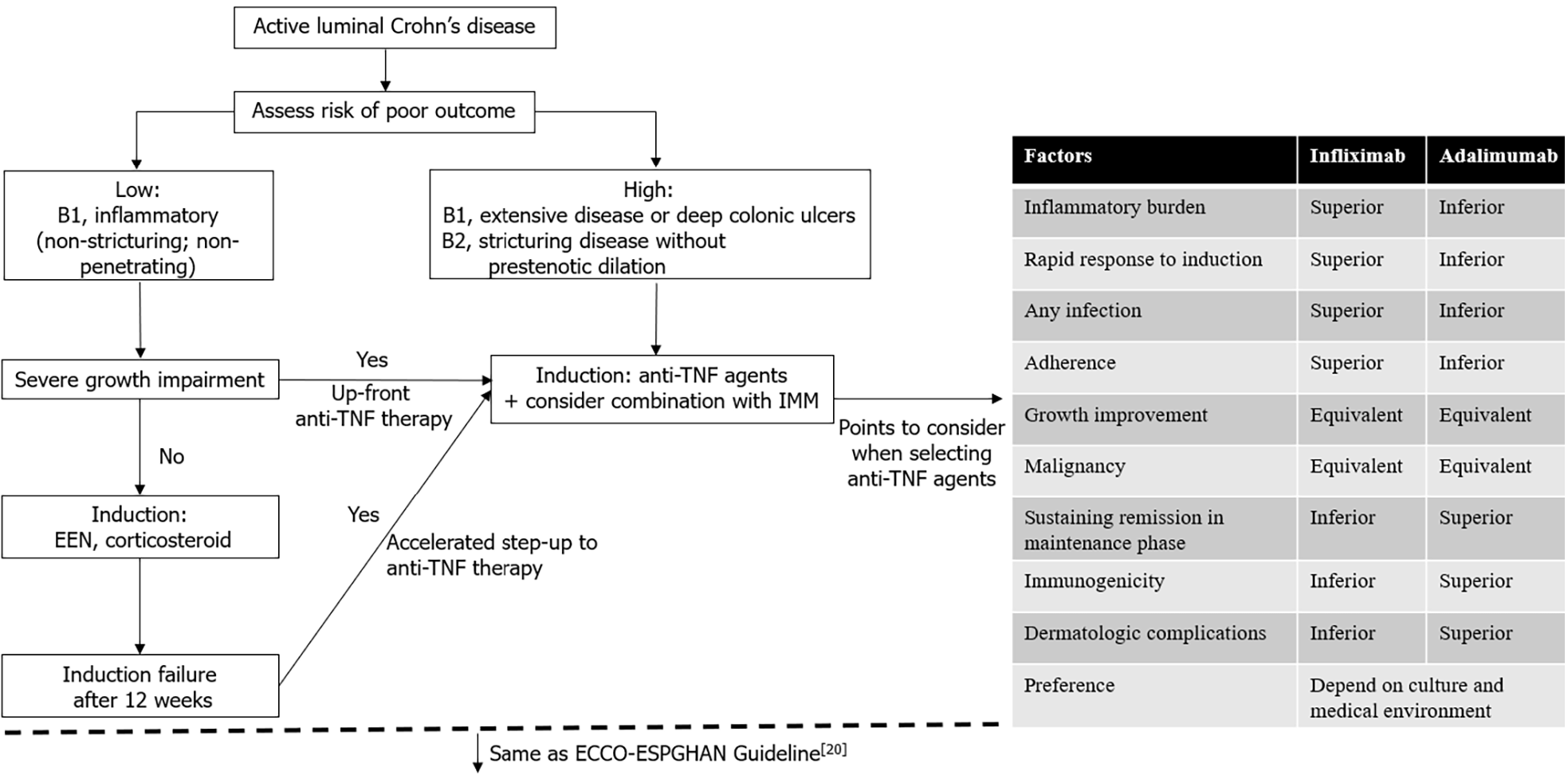
Figure 2 Summary flowchart of medical management of pediatric luminal Crohn’s disease and points to consider when selecting anti-tumor necrosis factor agents.
TNF: Tumor necrosis factor; EEN: Exclusive enteral nutrition; IMM: Immunomodulators. Citation: van Rheenen PF, Aloi M, Assa A, Bronsky J, Escher JC, Fagerberg UL, Gasparetto M, Gerasimidis K, Griffiths A, Henderson P, Koletzko S, Kolho KL, Levine A, van Limbergen J, Martin de Carpi FJ, Navas-López VM, Oliva S, de Ridder L, Russell RK, Shouval D, Spinelli A, Turner D, Wilson D, Wine E, Ruemmele FM. The Medical Management of Paediatric Crohn's Disease: an ECCO-ESPGHAN Guideline Update. J Crohns Colitis 2020. Copyright ©Oxford University Press 2020. Published by Oxford University Press[20].
- Citation: Kim ES, Kang B. Infliximab vs adalimumab: Points to consider when selecting anti-tumor necrosis factor agents in pediatric patients with Crohn’s disease. World J Gastroenterol 2023; 29(18): 2784-2797
- URL: https://www.wjgnet.com/1007-9327/full/v29/i18/2784.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i18.2784