Copyright
©The Author(s) 2023.
World J Gastroenterol. Apr 14, 2023; 29(14): 2202-2221
Published online Apr 14, 2023. doi: 10.3748/wjg.v29.i14.2202
Published online Apr 14, 2023. doi: 10.3748/wjg.v29.i14.2202
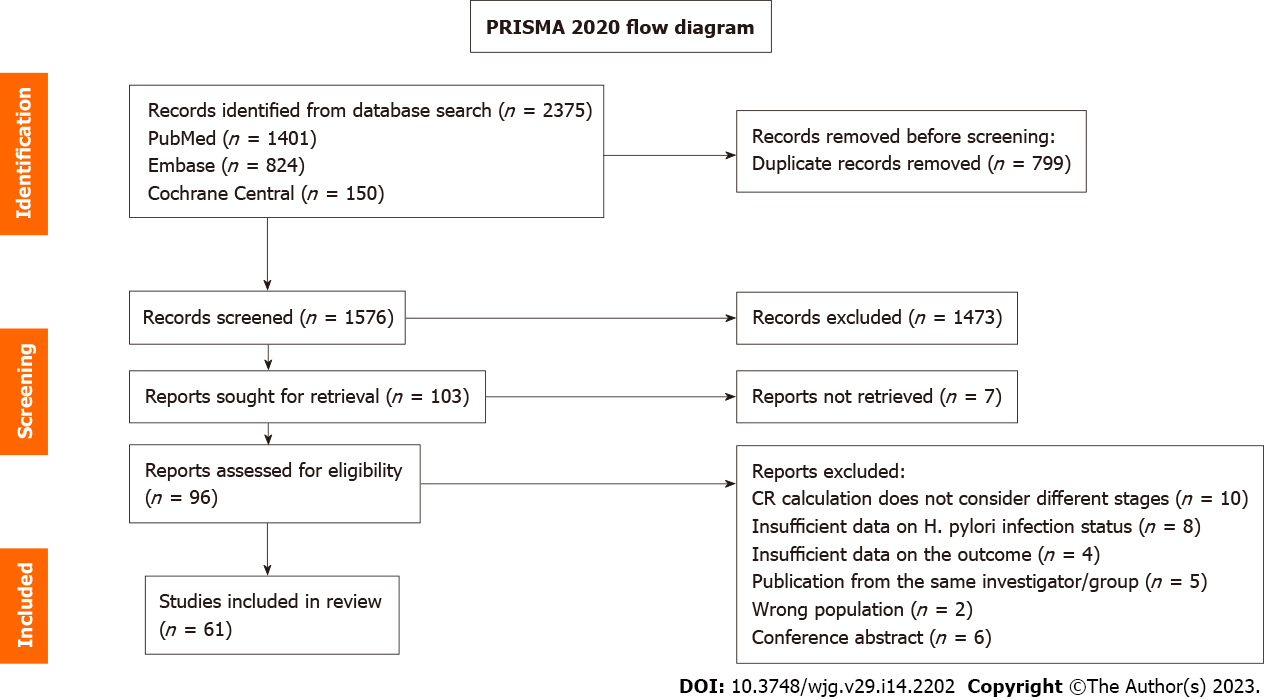
Figure 1 Preferred Reporting Items for Systematic Reviews and Meta-analyses 2020 flow diagram.
The flow chart describes the flow of information through the different stages of the systematic review and maps the number of records identified, included, and excluded, and the reasons for study exclusion. H. pylori: Helicobacter pylori.
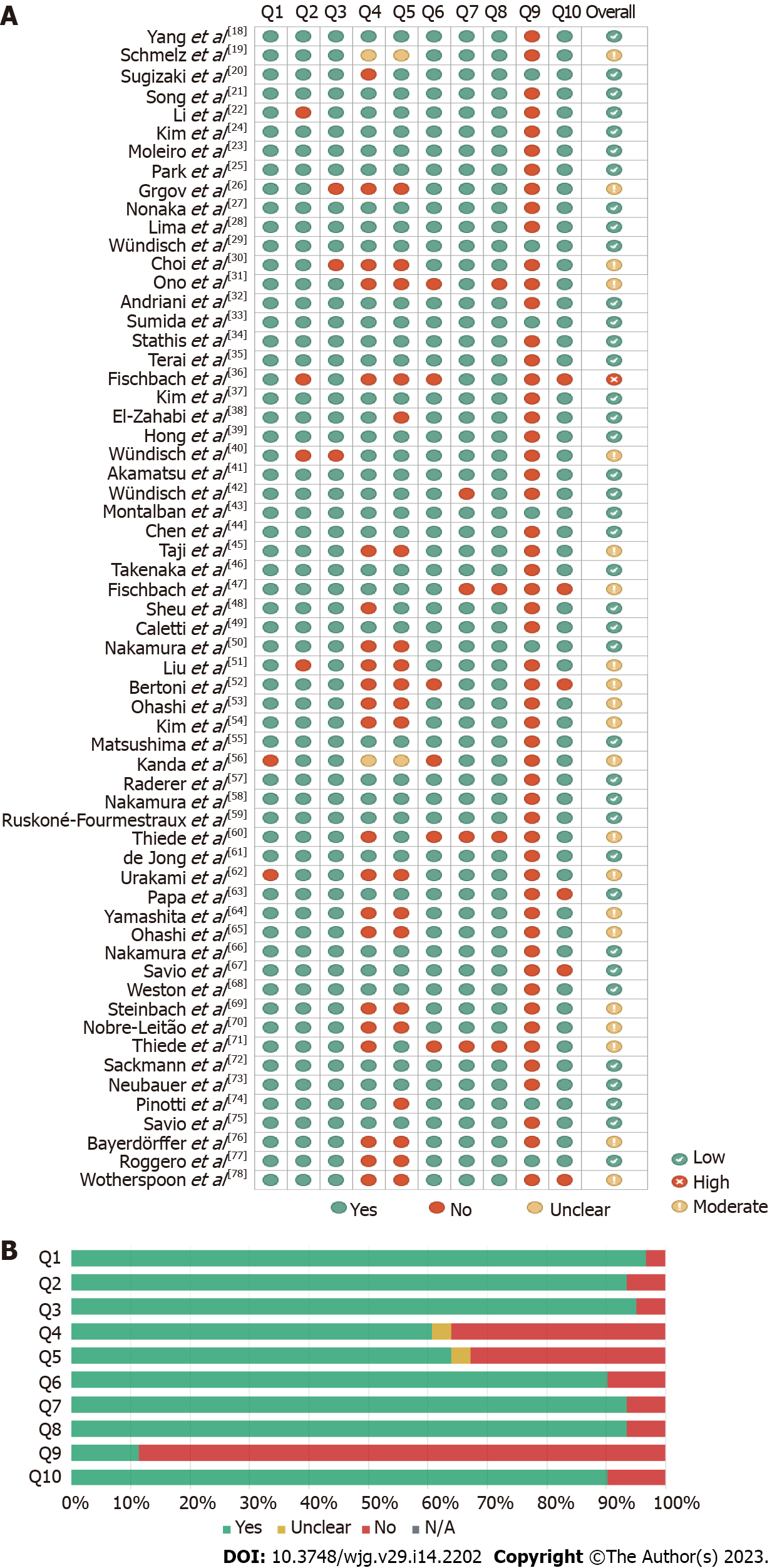
Figure 2 Risk of bias assessment by the Joanna Briggs Institute Critical Appraisal Tool.
The critical appraisal checklist for case series consists of 10 questions: Q1: Were there clear criteria for inclusion in the case series? Q2: Was the condition measured in a standard, reliable way for all participants included in the case series? Q3: Were valid methods used for identification of the condition for all participants included in the case series? Q4: Did the case series have consecutive inclusion of participants? Q5: Did the case series have complete inclusion of participants? Q6: Was there clear reporting of the demographics of the participants in the study? Q7: Was there clear reporting of clinical information of the participants? Q8: Were the outcomes or follow-up results of cases clearly reported? Q9: Was there clear reporting of the presenting site(s)/clinic(s) demographic information? Q10: Was statistical analysis appropriate? The percentage of risk of bias was calculated by the number of “yes” answers selected in the checklist. Questions with “not applicable” answers were not considered in the calculation. The risk of bias was classified using the following categories: High (scores up to 49.0%), moderate (scores between 50.0% and 70.0%), and low (scores above 70.0%). A: Overall risk of bias; B: Risk of bias summary: Discriminated assessments for each question across all studies. N/A: Not applicable.
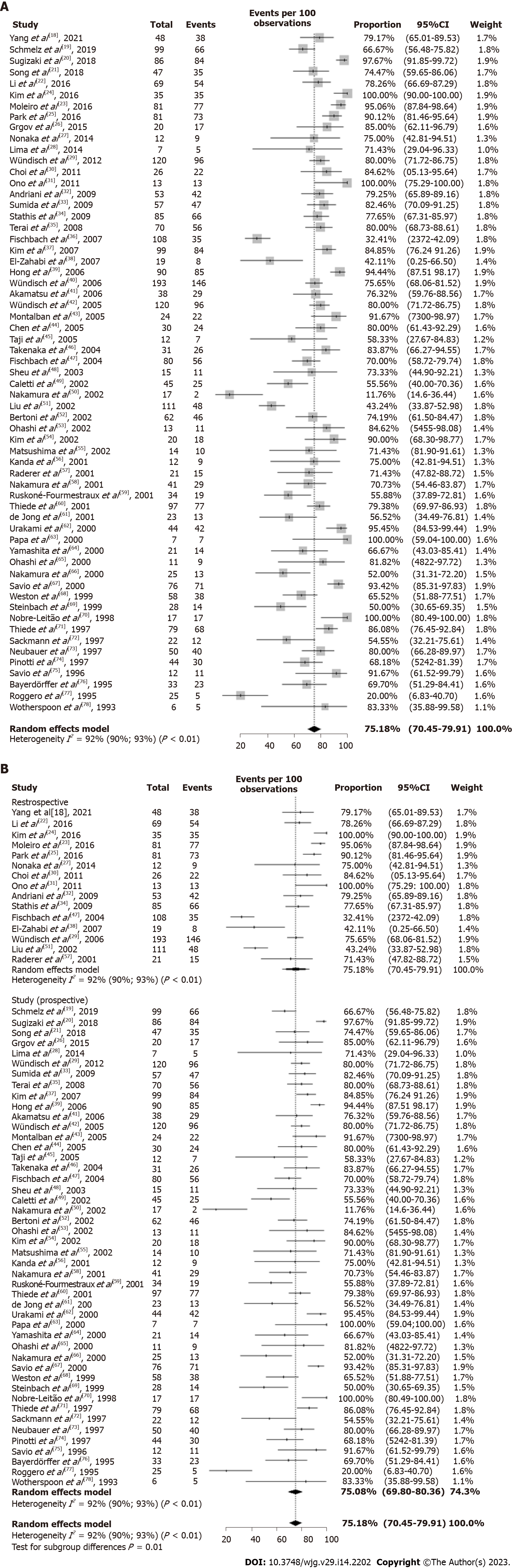
Figure 3 Overall complete remission rate of Helicobacter pylori-positive early-stage gastric mucosa-associated lymphoid tissue lymphoma.
A: After eradication therapy; B: After eradication therapy by study design. CI: Confidence interval.
- Citation: Lemos FFB, Castro CT, Calmon MS, Silva Luz M, Pinheiro SLR, Faria Souza Mendes dos Santos C, Correa Santos GL, Marques HS, Delgado HA, Teixeira KN, Souza CL, Oliveira MV, Freire de Melo F. Effectiveness of Helicobacter pylori eradication in the treatment of early-stage gastric mucosa-associated lymphoid tissue lymphoma: An up-to-date meta-analysis. World J Gastroenterol 2023; 29(14): 2202-2221
- URL: https://www.wjgnet.com/1007-9327/full/v29/i14/2202.htm
- DOI: https://dx.doi.org/10.3748/wjg.v29.i14.2202