Copyright
©The Author(s) 2022.
World J Gastroenterol. Dec 28, 2022; 28(48): 6875-6887
Published online Dec 28, 2022. doi: 10.3748/wjg.v28.i48.6875
Published online Dec 28, 2022. doi: 10.3748/wjg.v28.i48.6875
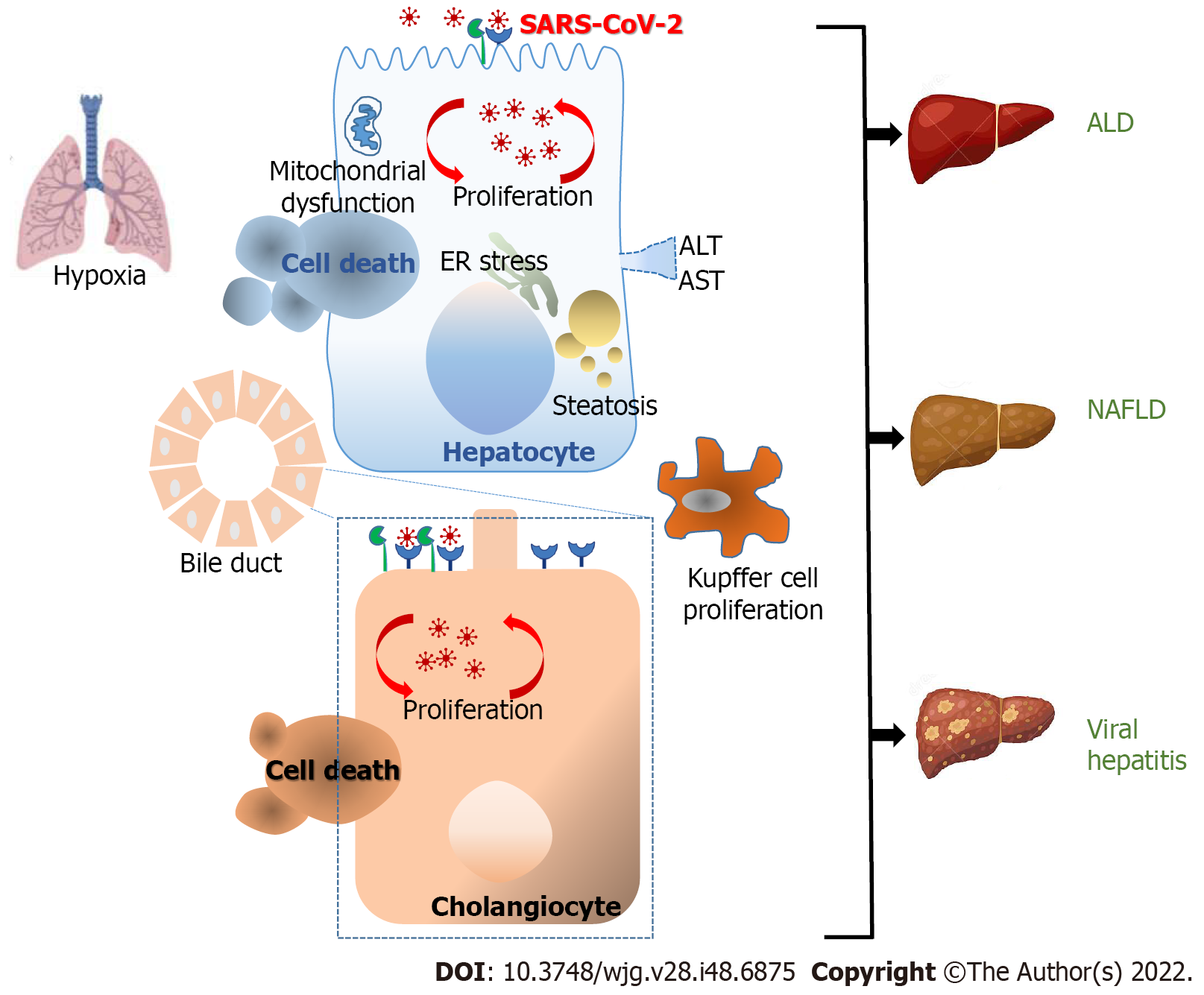
Figure 1 Mechanisms of pathological injury upon severe acute respiratory syndrome coronavirus 2 infection.
The major cause of mortality in coronavirus disease 2019 (COVID-19) is largely caused by lung damage with the increase of acute respiratory distress syndrome (ARDS). Liver damage or liver dysfunction has been linked with the general severity of COVID-19 infection and serves as a prognostic factor for ARDS progress. The scale of liver injury may range from direct severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral antigens, inflammatory progressions, hypoxemia, the antiviral treatments that induced hepatic damage and the existence of previous liver diseases. Angiotensin-converting enzyme 2 was mostly expressed in cholangiocytes and to a lesser extent in hepatocytes. These findings are in line with the viral presence reported in cholangiocytes and hepatocytes and the direct cytopathic effects observed. SARS-CoV-2 infection of hepatocytes and the indirect effects of “cytokine storm” induce a significant increase in alanine aminotransferase and aspartate aminotransferase indicating abnormal liver function. This leads to endoplasmic reticulum stress, steatosis, and finally to hepatocyte cell death. Consequently, Kupffer cell activation appears to be commonly funded in livers. The synergism between SARS-CoV-2 and chronic viral hepatitis B and C has also been suggested. Additionally, the superimposed cytokine storm caused by SARS-CoV-2 in patients with alcohol-associated liver disease, and non-alcoholic fatty liver disease (NALDF) displayed a higher risk of severe COVID-19. ALD: Alcohol-associated liver disease; ALT: Alanine aminotransferase; AST: Aspartate aminotransferase.
- Citation: Quarleri J, Delpino MV. Molecular mechanisms implicated in SARS-CoV-2 liver tropism. World J Gastroenterol 2022; 28(48): 6875-6887
- URL: https://www.wjgnet.com/1007-9327/full/v28/i48/6875.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i48.6875