Copyright
©The Author(s) 2022.
World J Gastroenterol. Jan 28, 2022; 28(4): 464-478
Published online Jan 28, 2022. doi: 10.3748/wjg.v28.i4.464
Published online Jan 28, 2022. doi: 10.3748/wjg.v28.i4.464
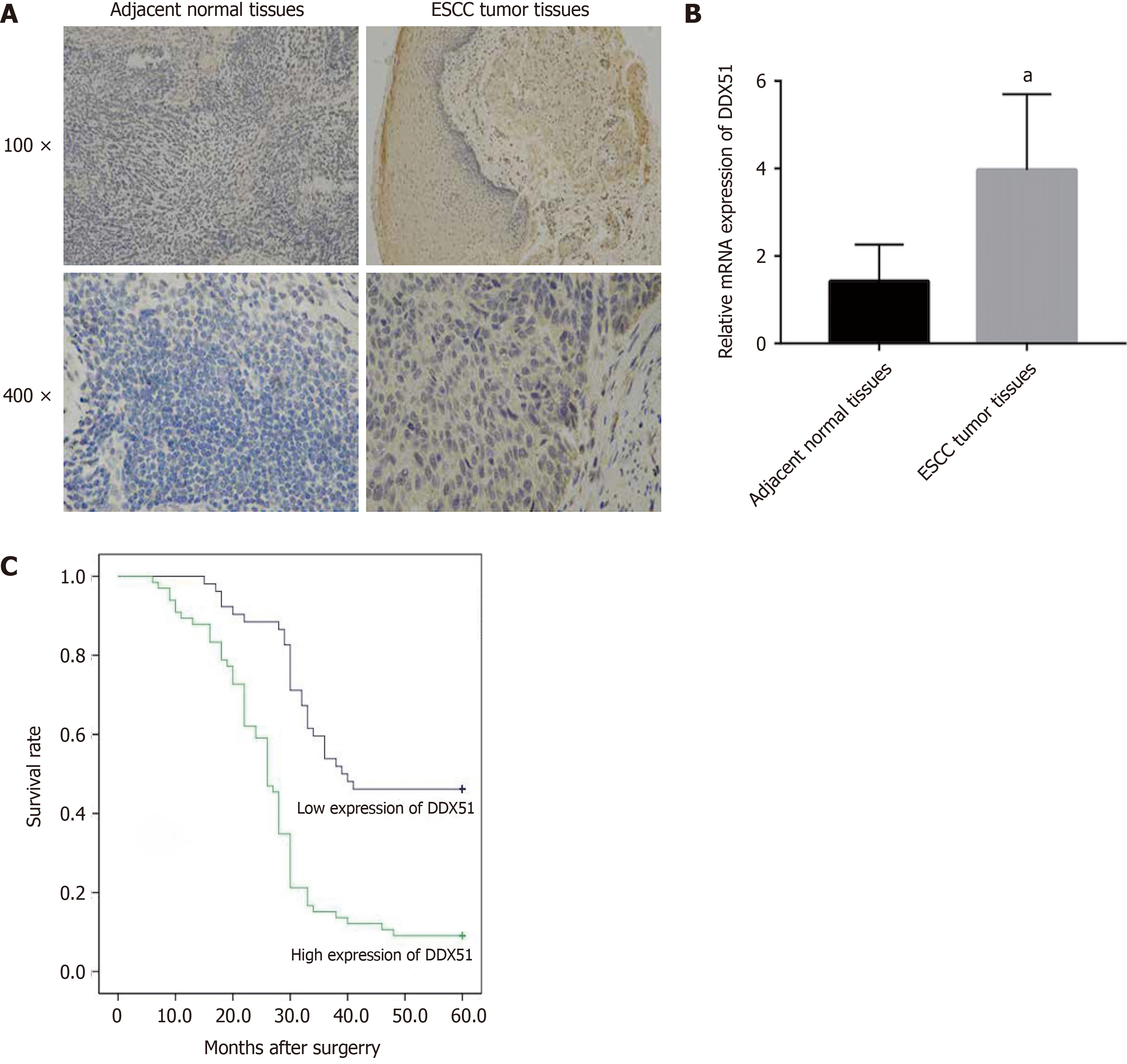
Figure 1 Expression and prognostic value of DEAD-box helicase 51 in esophageal cancer.
A: Immunohistochemistry analysis of DEAD-box helicase 51 (DDX51) expression in tumor tissues and paired normal tissues of patients with esophageal squamous cell carcinoma (ESCC); B: Quantitative PCR detection of DDX51 mRNA expression in the tumor tissues (n = 118) and paired normal tissues (n = 118) of patients with ESCC, aP < 0.05; C: Survival curves of ESCC patients with high or low expression (P = 0.0036).
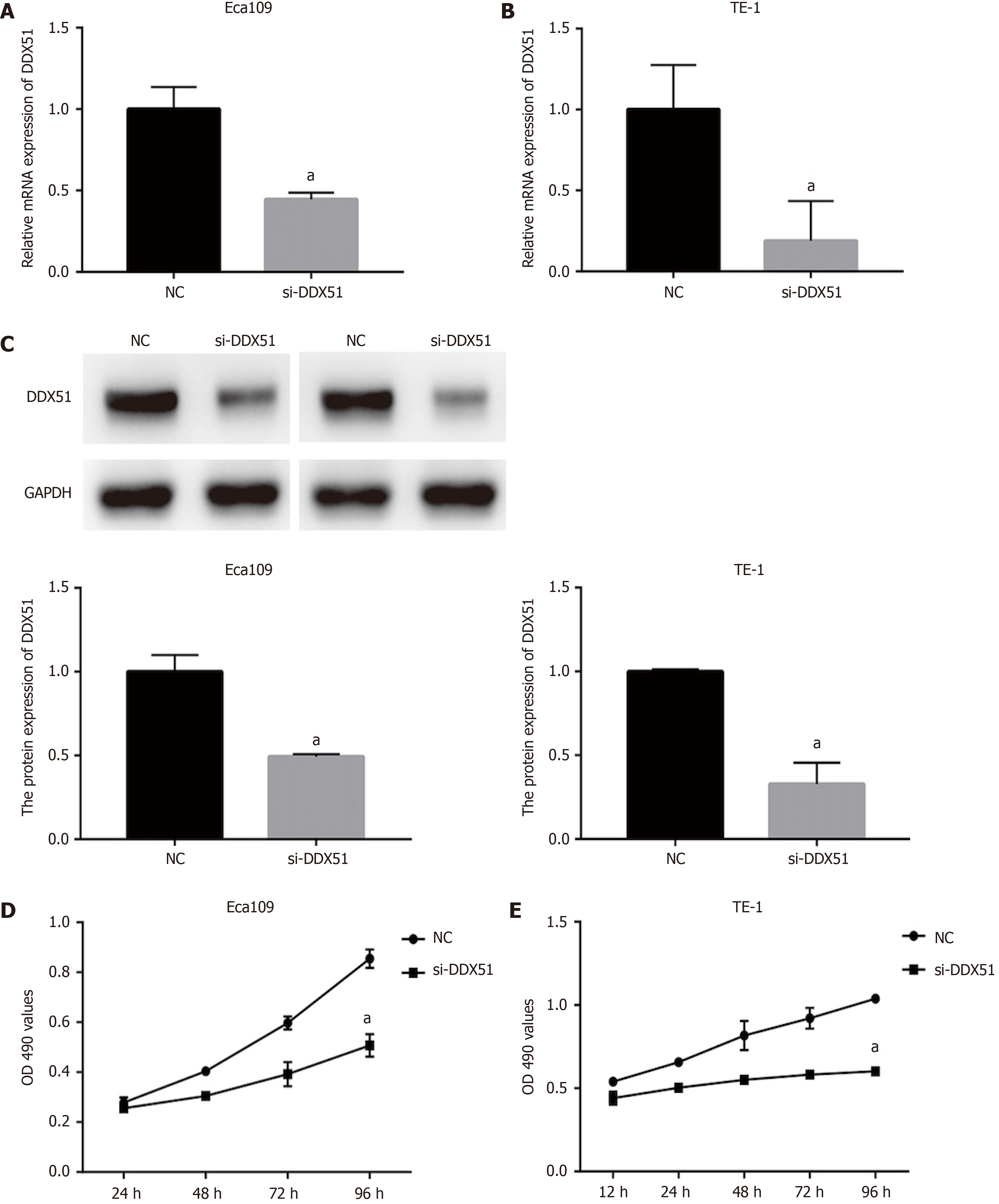
Figure 2 Anti-proliferation effects of DEAD-box helicase 51 in esophageal cancer.
Negative control (NC) small interfering RNA (siRNA) and DEAD-box helicase 51 (DDX51) siRNA were synthesized and transfected into Eca109 and TE-1 cells. A and B: Quantitative PCR assay was used for the detection of knockdown efficiencies; C: Western blot analysis of DDX51 expression in DDX51 siRNA-expressing cells; D and E: The effects of DDX51 on the viability of esophageal squamous cell carcinoma cells. aP < 0.05. All data were obtained from at least three independent experiments. NC was a scrambled siRNA.
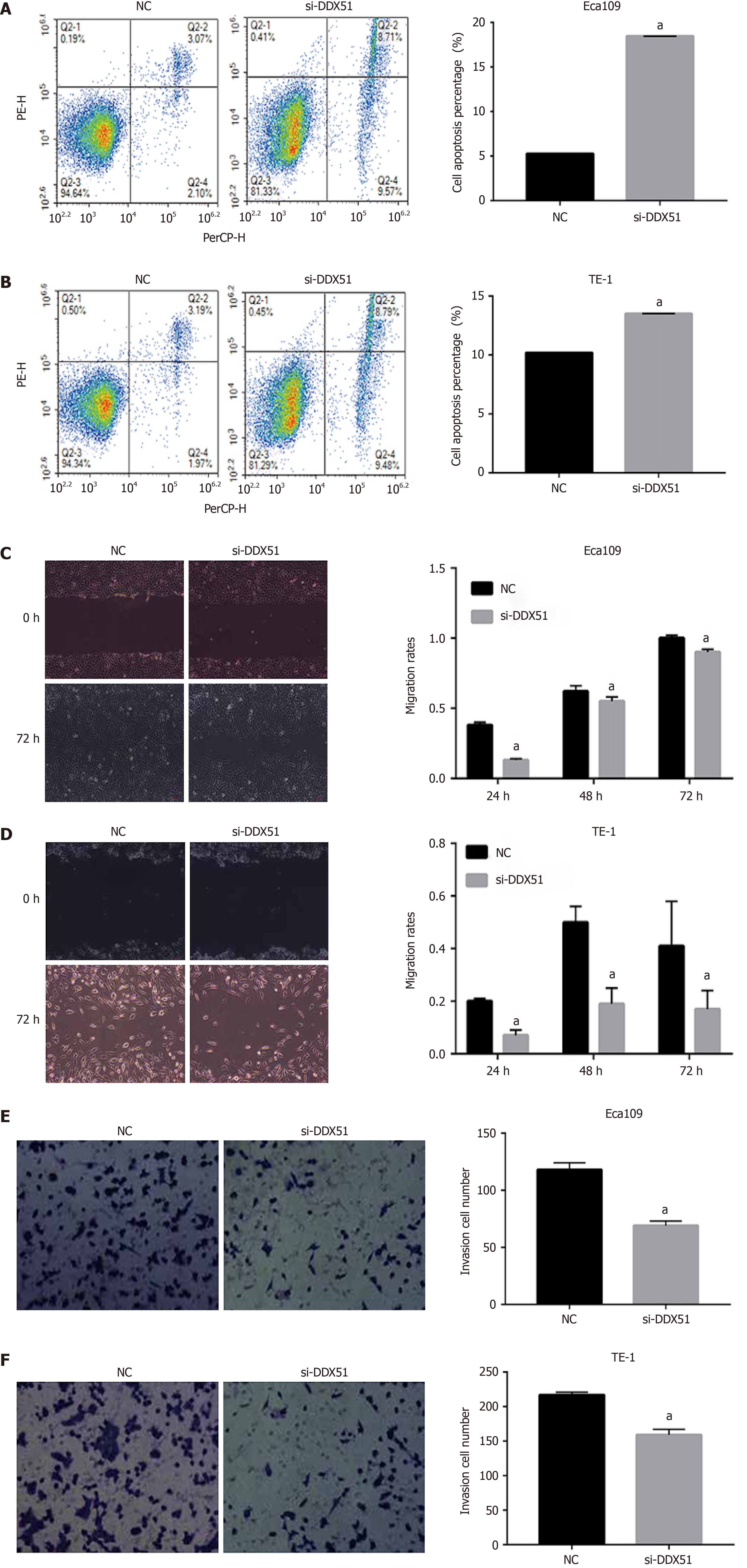
Figure 3 Effects of DEAD-box helicase 51 small interfering RNA on cell apoptosis, migration, and invasion of esophageal squamous cell carcinoma cells.
A and B: Cell apoptosis percentage of DEAD-box helicase 51 (DDX51) small interfering RNA (siRNA)-expressing esophageal squamous cell carcinoma (ESCC) cells; C and D: Scratch assays for cell migration analysis of the DDX51 siRNA-expressing ESCC cells; E and F: Transwell assays for cell invasion analysis of DDX51 siRNA-expressing ESCC cells. aP < 0.05. All data were obtained from at least three independent experiments. Negative control was a scrambled siRNA.
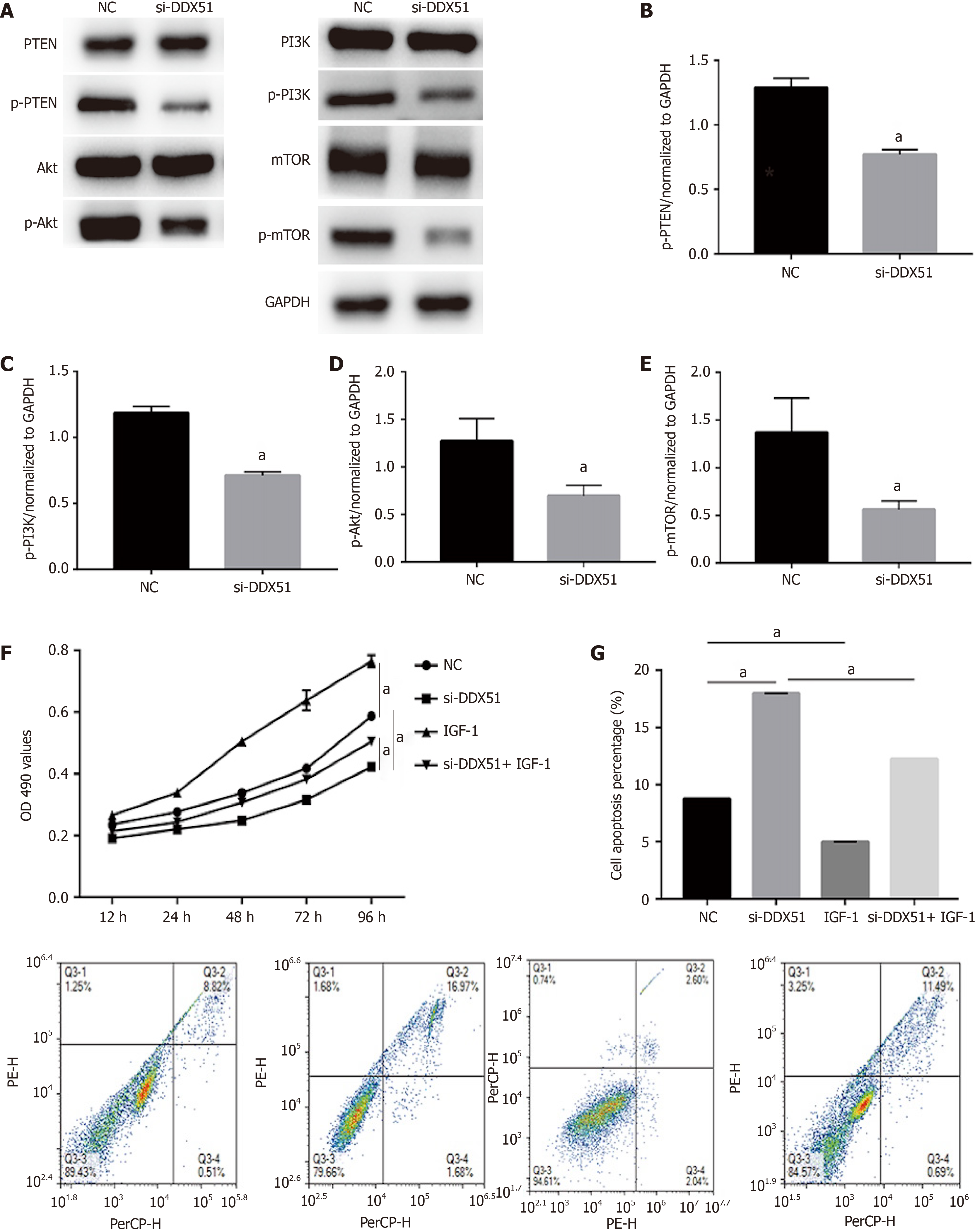
Figure 4 Knockdown of DEAD-box helicase 51 induced inactivation of the phosphoinositide 3-kinase/AKT signaling pathway.
A-E: Expression of phosphoinositide 3-kinase (PI3K)/AKT pathway members, including phosphatase and tenin homolog (PTEN), phosphorylated PTEN (p-PTEN), PI3K, p-PI3K, AKT, p-AKT, mammalian target of rapamycin (mTOR) and p-mTOR, was detected by western blot analysis; F: AKT activator insulin-like growth factor 1 (IGF-1) reversed the inhibitory effect of DDX51 knockdown on the proliferation of TE-1 cells; G: AKT activator IGF-1 reversed the pro-apoptotic effect of DDX51 knockdown on the proliferation of TE-1 cells. aP < 0.05. All data were obtained from at least three independent experiments. Negative control was a scrambled small interfering RNA.
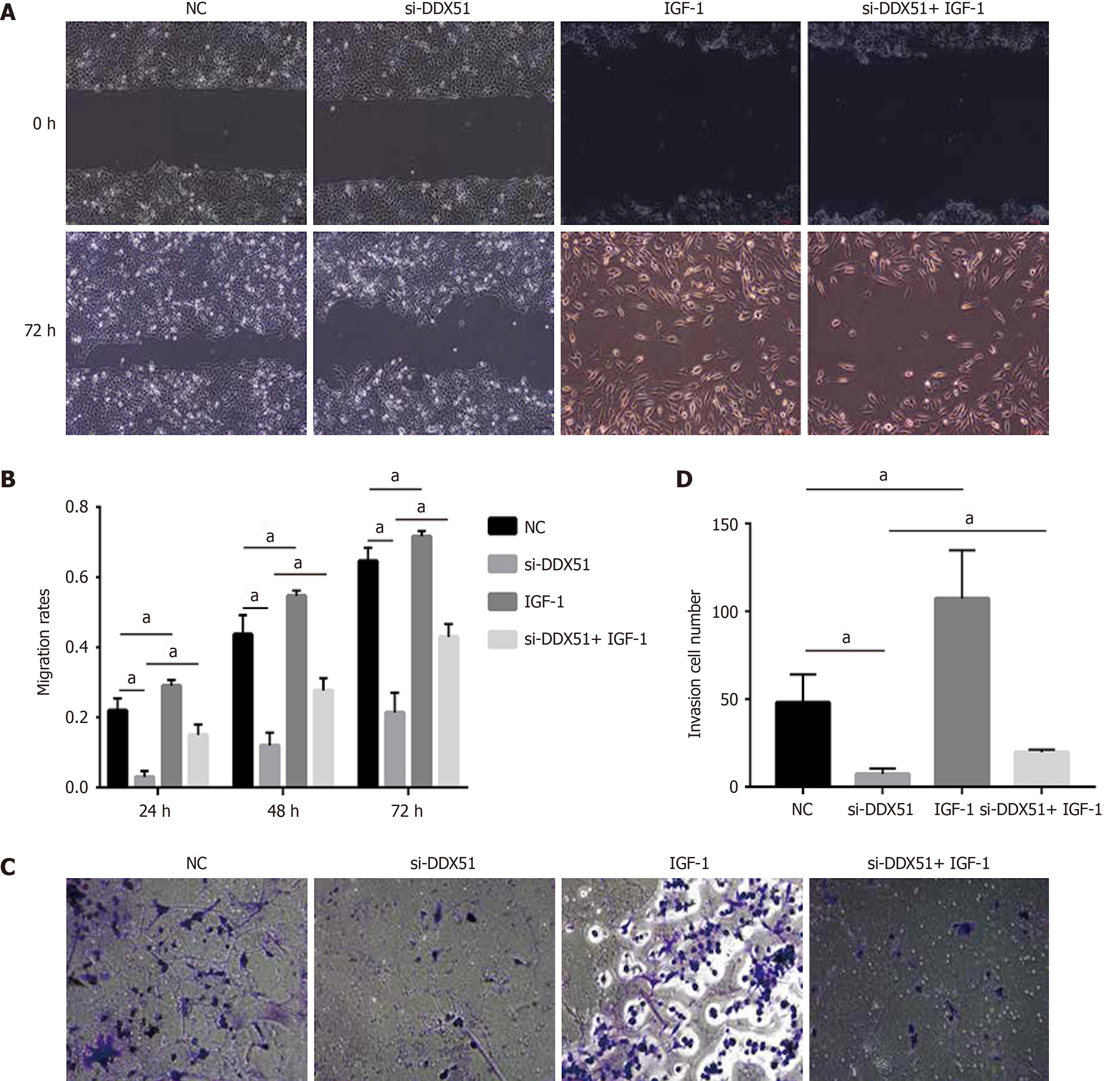
Figure 5 Anti-migration and anti-invasion effects of DEAD-box helicase 51 knockdown was mediated by the AKT pathway.
A and B: AKT activator insulin-like growth factor 1 (IGF-1) reversed the anti-migration effect of DDX51 knockdown on the proliferation of TE-1 cells; C and D: AKT activator IGF-1 reversed the anti-invasion effect of DDX51 knockdown on the proliferation of TE-1 cells. aP < 0.05. All data were obtained from at least three independent experiments. Negative control was a scrambled small interfering RNA.
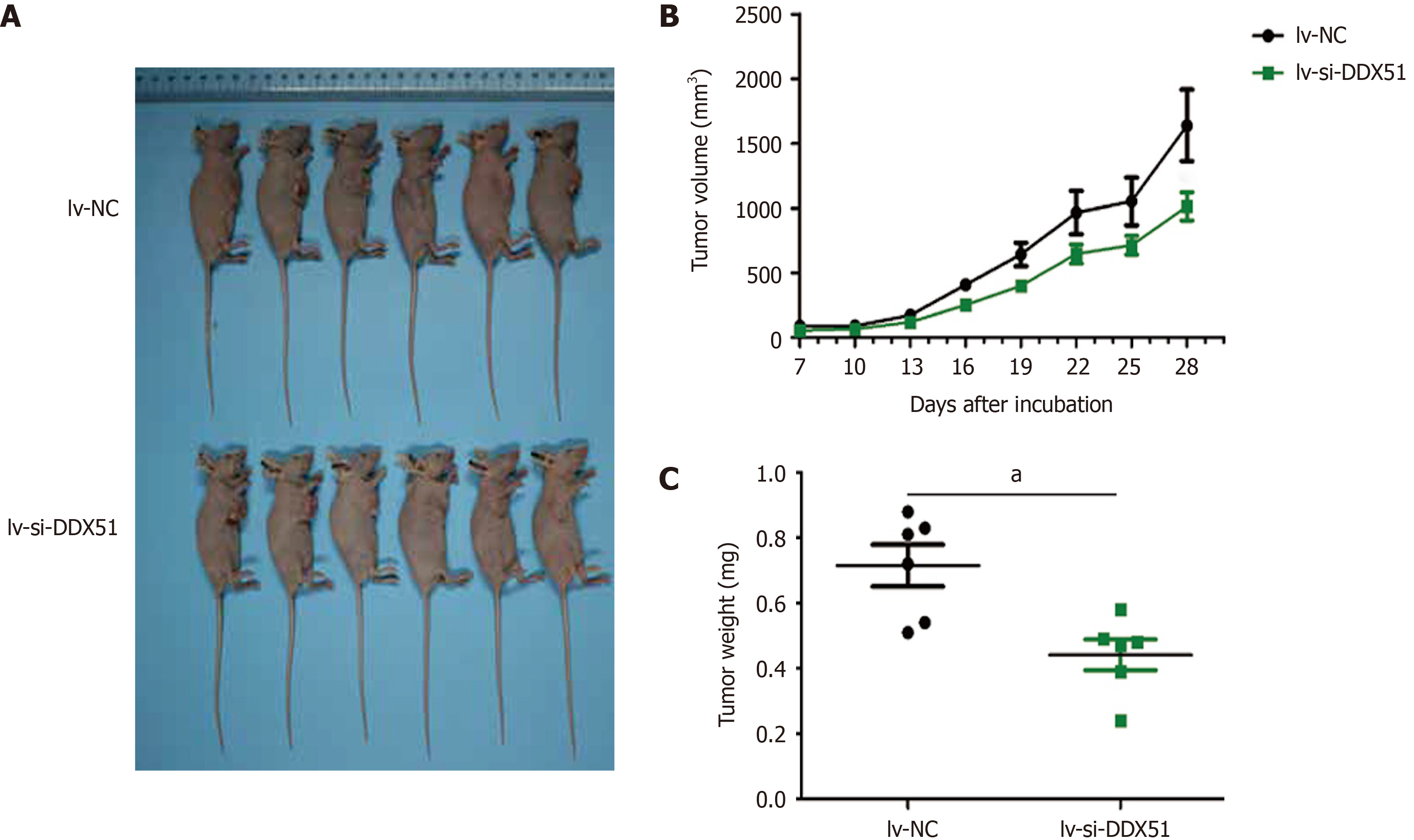
Figure 6 DEAD-Box helicase 51 knockdown inhibited the tumor growth of esophageal squamous cell carcinoma in vivo.
TE-1 cells transfected with lentivirus-small interfering-negative control (lv-si-NC) (n = 6) or lv-si-DEAD-box helicase 51 (DDX51) (n = 6) were injected into nude mice and tumor volumes were monitored. A: The photographs of mice with esophageal squamous cell carcinoma tumor of TE-1 cells transfected with lv-si-NC or lv-si-DDX51; B: Tumor growth curves; C: At the end of experiments, mice were executed, tumors were excised and tumor weights were measured. aP < 0.05. Negative control was a scrambled siRNA.
- Citation: Hu DX, Sun QF, Xu L, Lu HD, Zhang F, Li ZM, Zhang MY. Knockdown of DEAD-box 51 inhibits tumor growth of esophageal squamous cell carcinoma via the PI3K/AKT pathway. World J Gastroenterol 2022; 28(4): 464-478
- URL: https://www.wjgnet.com/1007-9327/full/v28/i4/464.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i4.464