Copyright
©The Author(s) 2022.
World J Gastroenterol. Sep 28, 2022; 28(36): 5265-5279
Published online Sep 28, 2022. doi: 10.3748/wjg.v28.i36.5265
Published online Sep 28, 2022. doi: 10.3748/wjg.v28.i36.5265
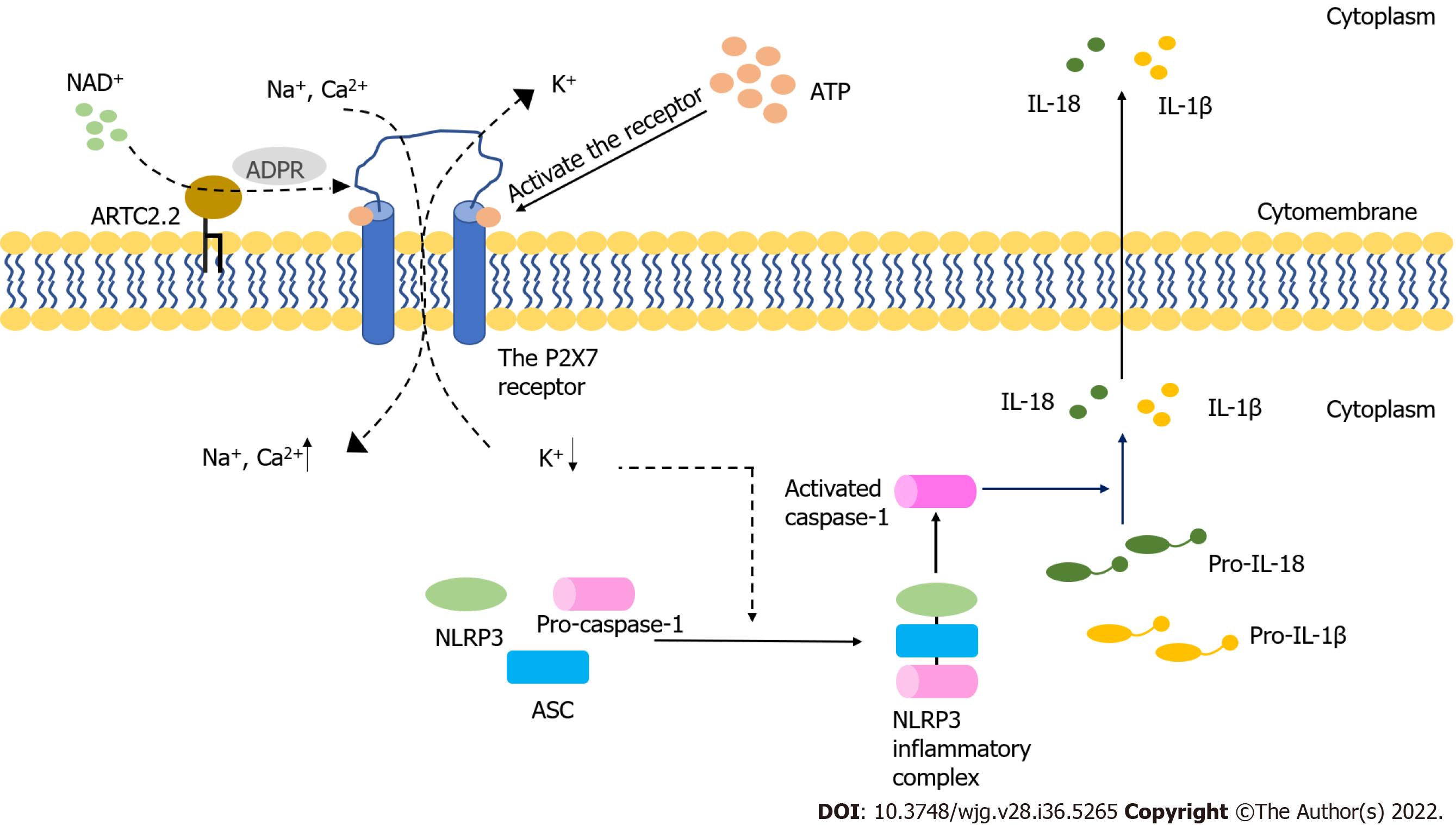
Figure 1 The activation of the P2X7 receptor and NLRP3 inflammasome.
The P2X7 receptor is activated by extracellular ATP and NAD+ and serves as an ion channel. It can also form non-selective macropores. The activated P2X7 receptor induces the decreasing of intracellular K+, which initiates NOD-like receptor family pyrin domain containing 3 (NLRP3) inflammasome activation. The activated process of pro-interleukin (IL)-1β and pro-IL-18 are triggered by the active caspase-1 that results from the formation of NLRP3 inflammasome. Mature inflammatory cytokines are released into extracellular space from cells, which finally results in the cell death. ADPR: ADP-ribose; ARTC2.2: ADP-ribosyltransferase 2.2; ASC: Apoptosis-associated speck-like protein containing a CARD; NLRP3: NOD-like receptor family pyrin domain containing 3; IL-18: Interleukin-18; IL-1β: Interleukin-1β.
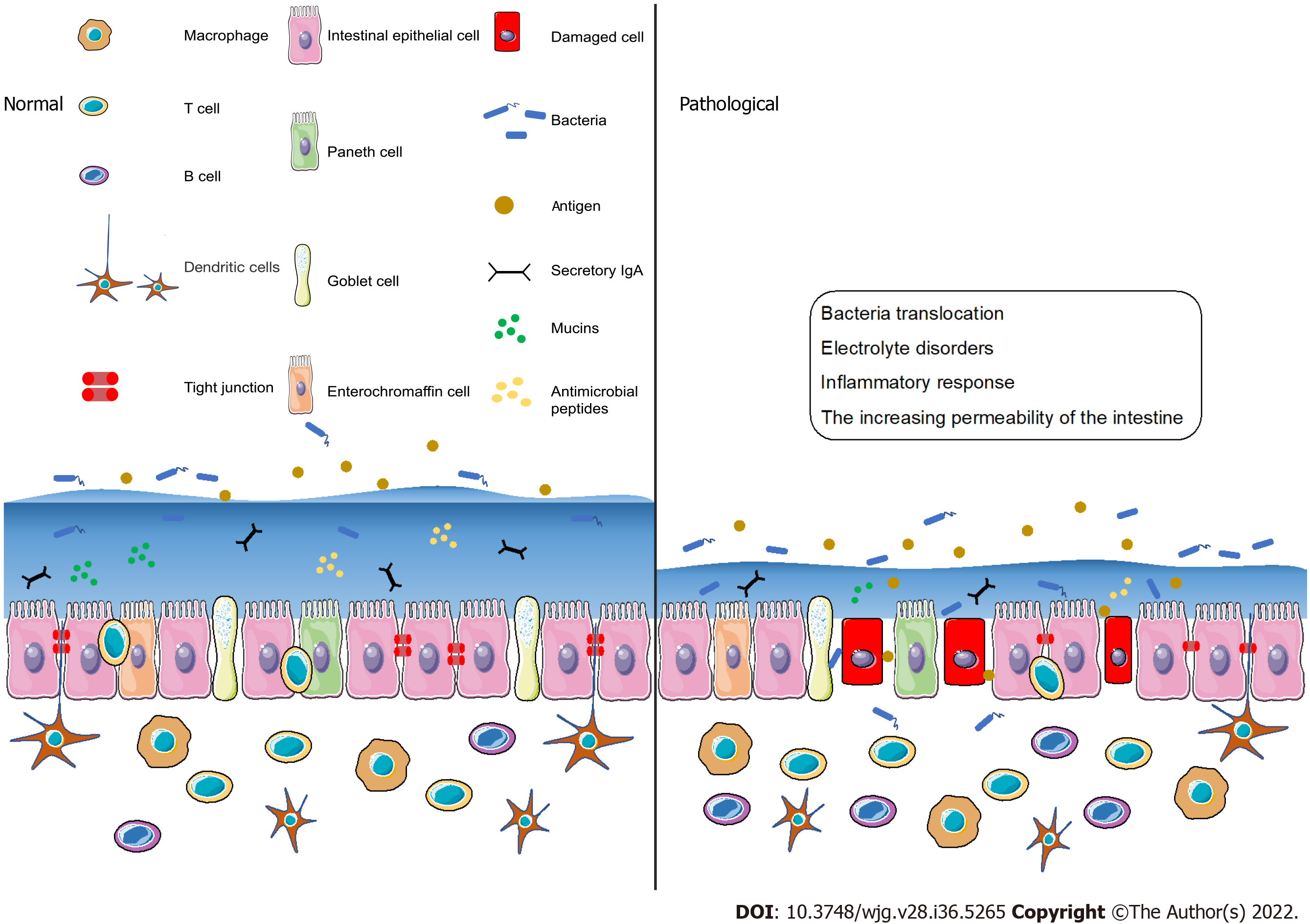
Figure 2 Intestinal barrier components and the intestinal barrier dysfunction.
The normal intestinal barrier is formed by many layers which includes cytokines, bacteria, cells and secretory IgA. The intestinal barrier dysfunction results in the increasing of the intestinal permeability, which subsequently causes the inflammatory response and bacteria translocation.
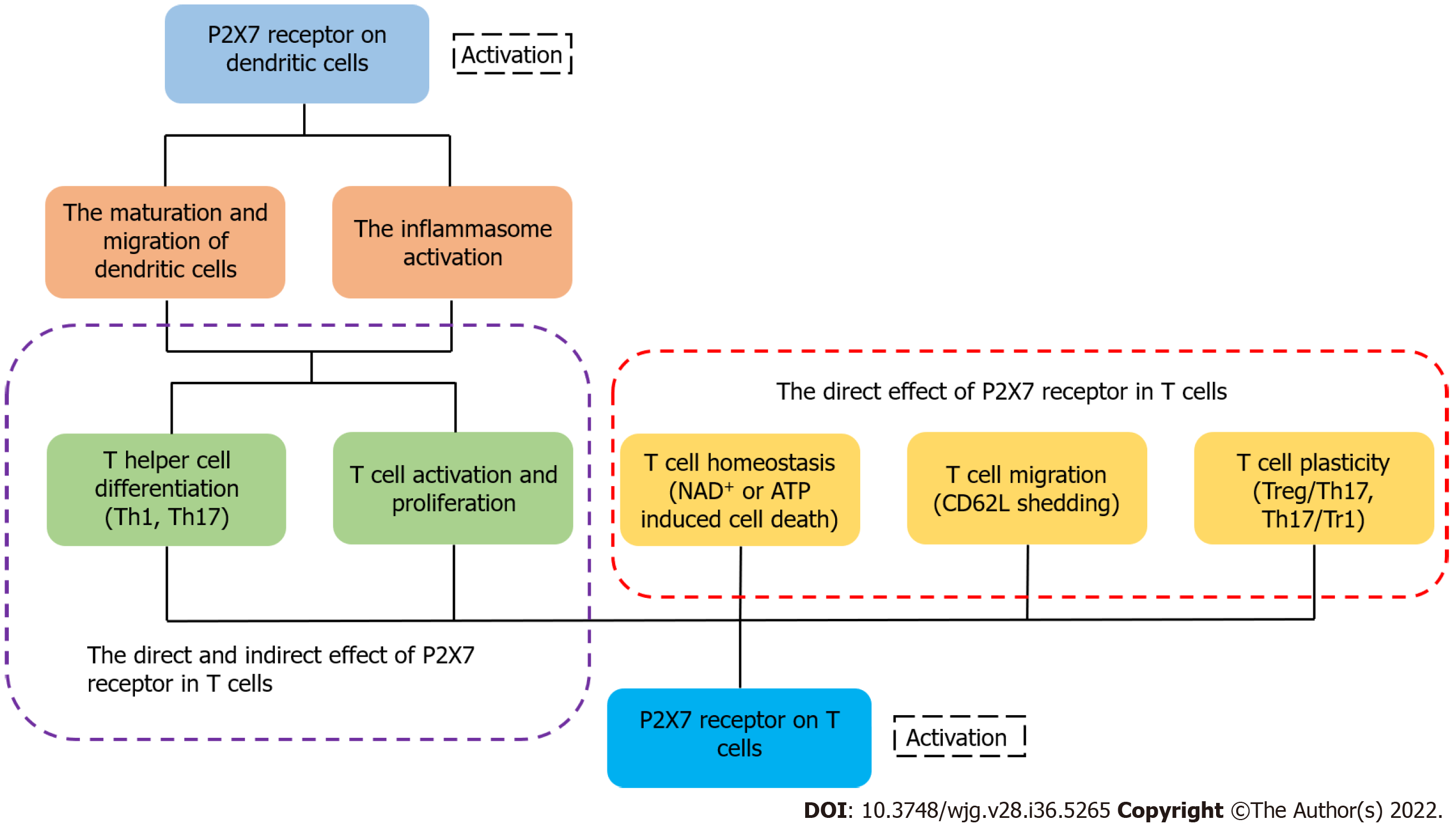
Figure 3 P2X7 receptor on dendritic cells and T cells influences the fate of T cells indirectly or directly, respectively.
The activation of P2X7 receptor on dendritic cells induces the maturation and migration of cells and promotes the inflammasome activation, affecting the activation and differentiation of T cells finally. The activation of P2X7 receptor in T cells can directly regulate the activation, differentiation, migration and homeostasis of T cells[33].
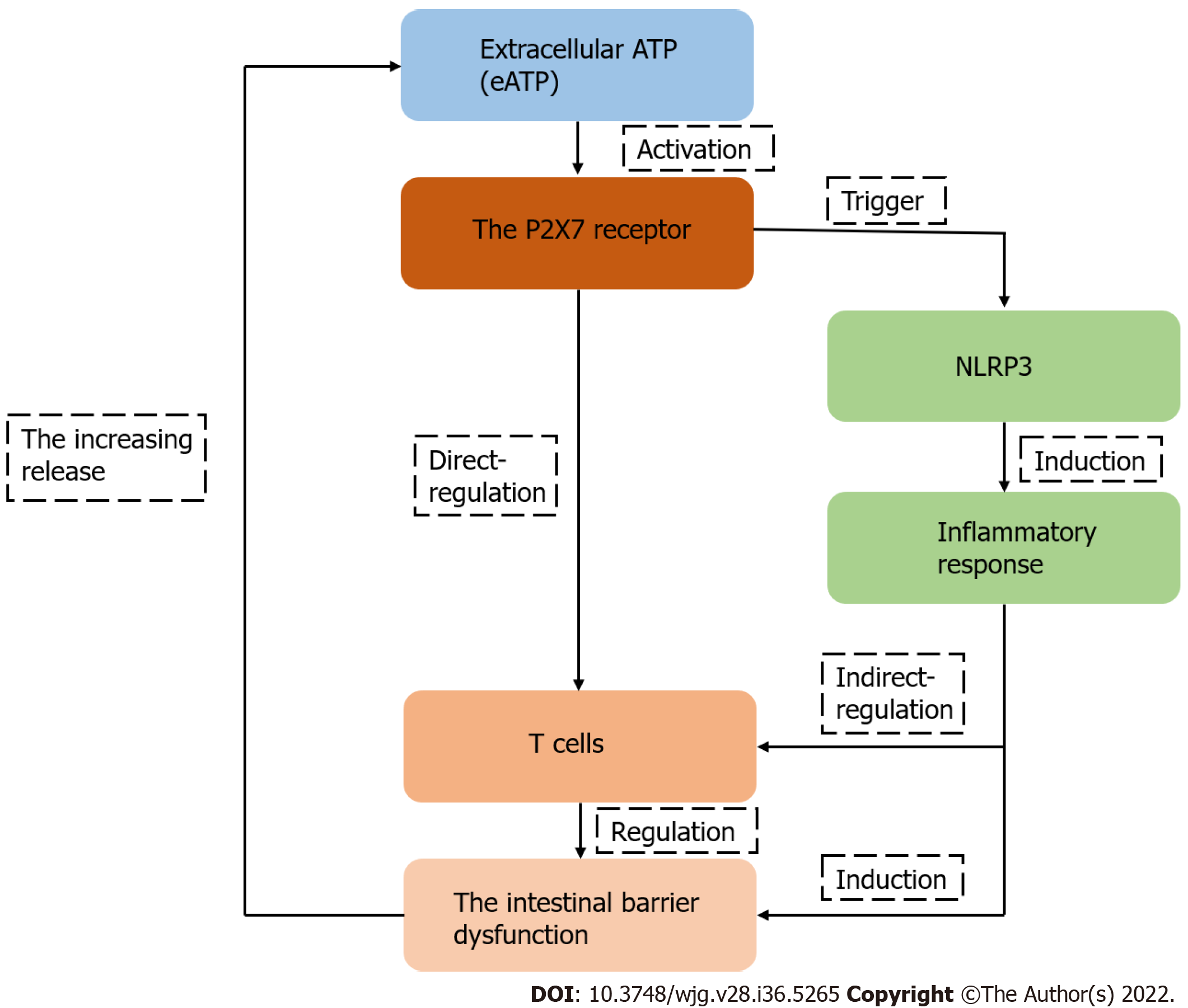
Figure 4 The schematic diagram of the hypothesis of the P2X7 receptor as the regulator of T-cell function in intestinal barrier disruption.
- Citation: Jiang ZF, Wu W, Hu HB, Li ZY, Zhong M, Zhang L. P2X7 receptor as the regulator of T-cell function in intestinal barrier disruption. World J Gastroenterol 2022; 28(36): 5265-5279
- URL: https://www.wjgnet.com/1007-9327/full/v28/i36/5265.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i36.5265