Copyright
©The Author(s) 2022.
World J Gastroenterol. Aug 21, 2022; 28(31): 4263-4298
Published online Aug 21, 2022. doi: 10.3748/wjg.v28.i31.4263
Published online Aug 21, 2022. doi: 10.3748/wjg.v28.i31.4263
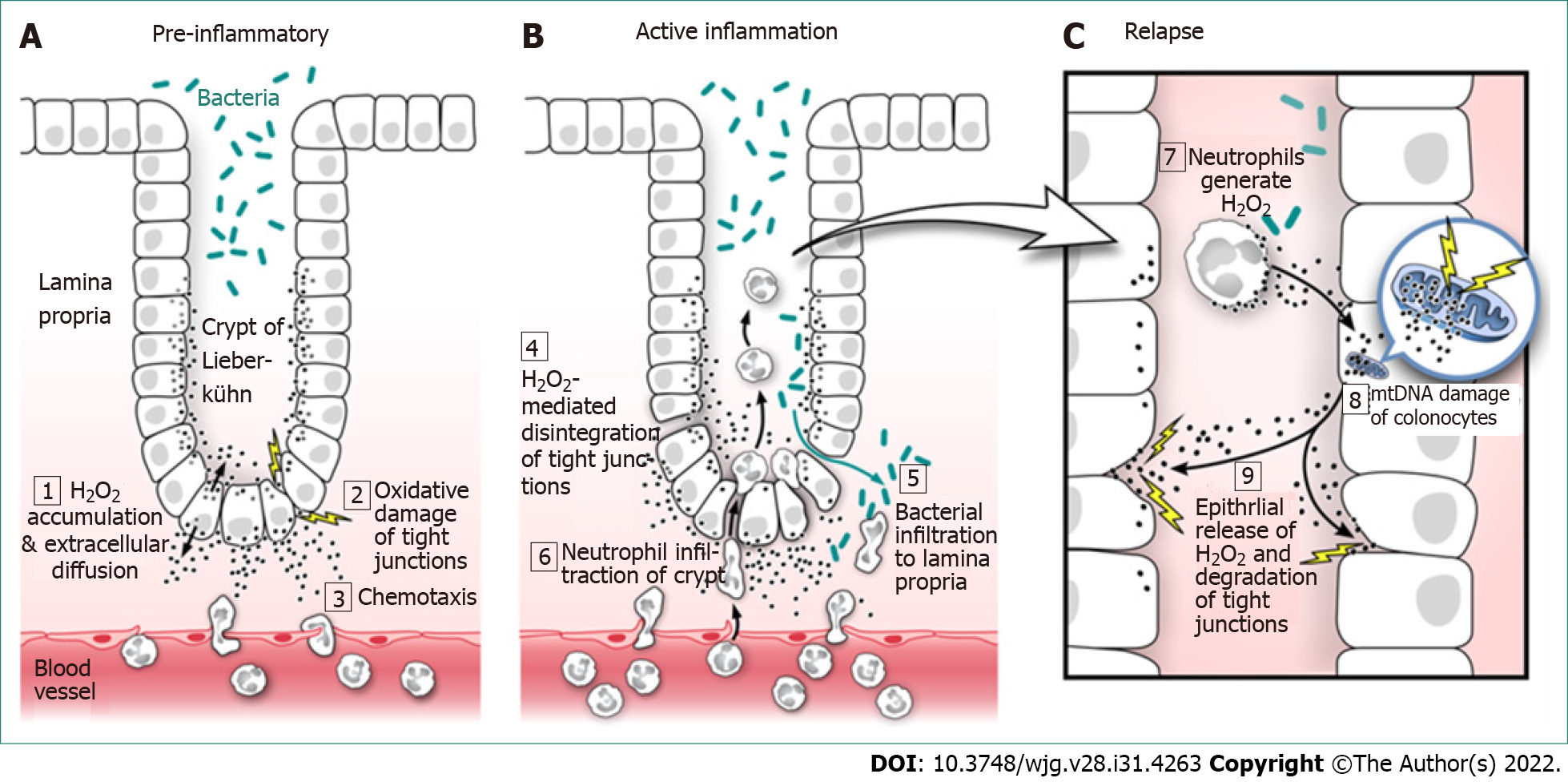
Figure 1 Ulcerative colitis: Evidence-based pathogenesis and relapse.
A: Pre-inflammatory; B: Active inflammation; C: Relapse. Hydrogen peroxide (H2O2) is produced by all cells of the body, mainly as a toxic by-product of cellular metabolism and must be immediately neutralized to prevent cell damage. If produced in excess by colonocytes (colonic epithelial cells), H2O2 easily diffuses through the cell membrane; 1: To the extracellular space where its unique properties of long life, potent oxidizing power, and the ability to attract neutrophils (neutrophilic chemotaxis) combine to promote oxidative damage of colonocyte tight junctions; 2: While attracting neutrophils into the colonic epithelium; 3: Continued H2O2 exposure leads to oxidative disintegration of tight junctional proteins and increased colonic epithelial paracellular permeability; 4: Increased paracellular permeability promotes bacterial translocation into the sterile lamina propria; 5: And facilitates neutrophil migration up the H2O2 concentration gradient into the crypts of Lieberkühn; 6: Both of which lead to colonic inflammation and eventual ulcerative colitis. Neutrophils exposed to bacteria in the crypts become activated and produce large amounts of H2O2 that diffuses into colonic epithelial cells; 7: Which adds to the already high colonocyte H2O2 load. The increased colonocyte H2O2 oxidizes mitochondrial DNA (mtDNA) introducing genetic mutations that miscode when transcribing for electron transport chain (ETC) proteins; 8: Faulty ETC proteins exhibit additional electron leakage leading to greater H2O2 production creating a vicious cycle of mtDNA damage and ever greater H2O2 production, which contributes to and increases the frequency and severity of relapse; 9: This amounts to a “hard-wired” genetic reprogramming that promotes colonic inflammation as discussed below. H2O2: Hydrogen peroxide; mtDNA: Mitochondrial DNA.
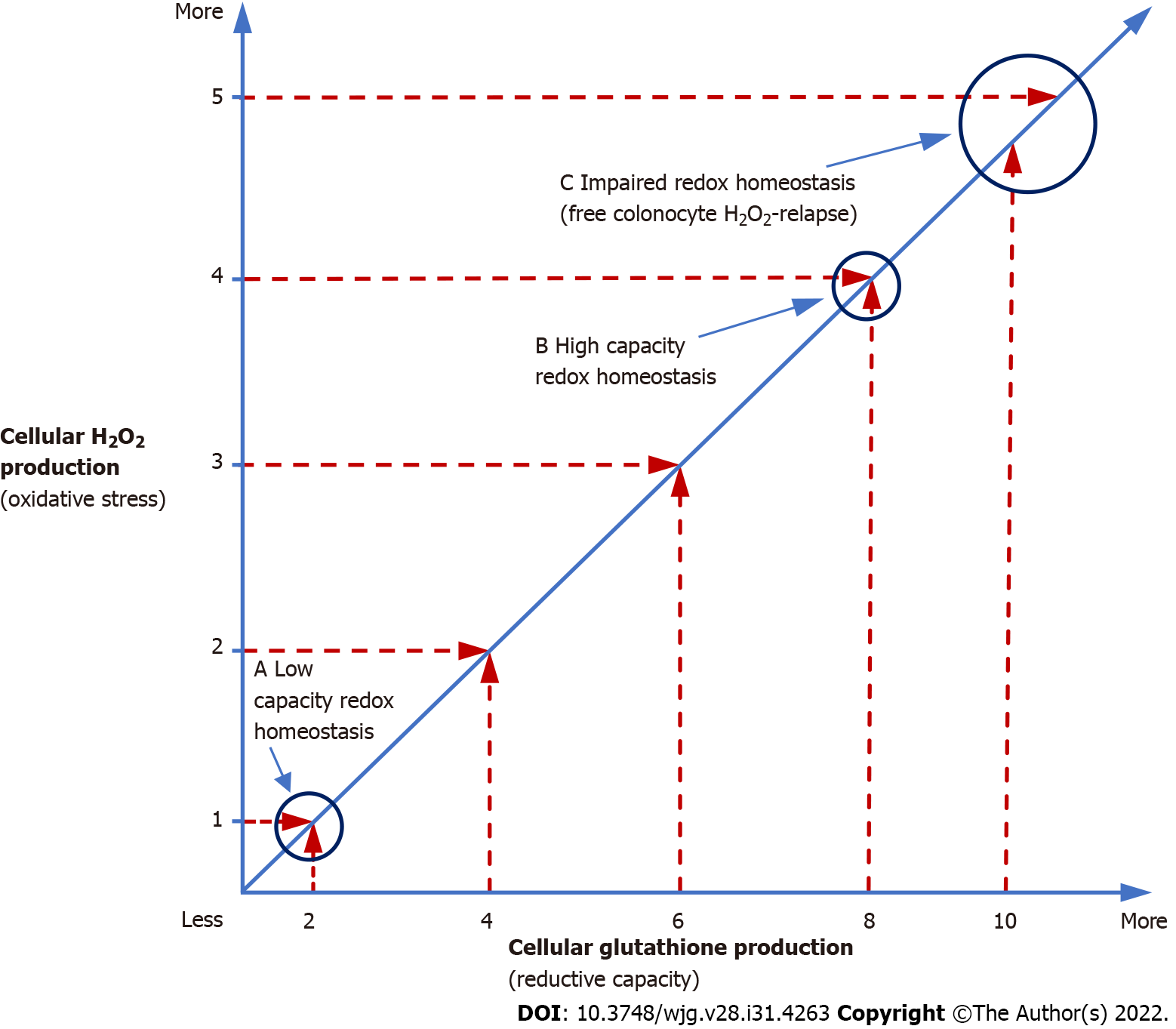
Figure 2 Redox homeostasis.
Redox homeostasis is more than just a balance between oxidizing [hydrogen peroxide (H2O2)] and reducing agents (glutathione). In the above graph, redox homeostasis (slanted line) is maintained at both low and high H2O2 production rates (a and b), but the cell is functioning at a higher oxidative capacity (high capacity redox homeostasis) (b), when more H2O2 is being produced compared to times when lesser amounts of H2O2 are being generated (a). Mitochondria, the site of most cellular H2O2 production, do not synthesize their own glutathione and only contain 10% of the total cellular supply of this vital reducing equivalent that must be generated in the cytoplasm and imported into mitochondria, which takes time[53]. Once depleted, mitochondrial glutathione can take several hours to restore to normal levels[46]. In contrast to the limited supply of mitochondrial glutathione, studies have shown that mitochondrial electron transport chain production of H2O2 can increase up to 15 × during periods of high metabolic demand[54]. Any increase in H2O2 production forces the cell to utilize additional glutathione in order to maintain redox balance which may lead to high capacity redox homeostasis (b). Since about 30% of cell thiols (i.e., glutathione) normally undergo oxidation per hour[55], the additional oxidative stress imposed by high capacity redox homeostasis can, over time, deplete available glutathione and overwhelm colonocyte reductive capacity creating a state of impaired redox homeostasis (c) followed by H2O2 build-up and extracellular diffusion, which can lead to de novo ulcerative colitis or relapse. High capacity redox homeostasis is consistent with increased H2O2 production observed in the non-inflamed ascending colonic epithelium of individuals with ulcerative colitis[30]. H2O2: Hydrogen peroxide.
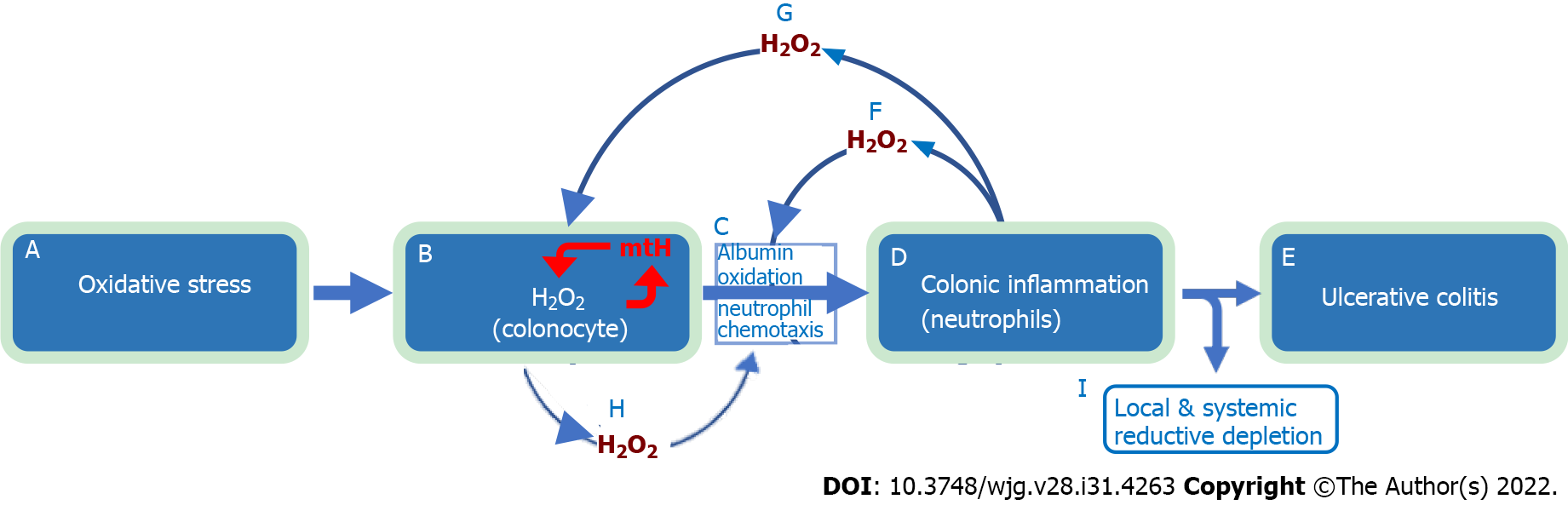
Figure 3 Natural history of ulcerative colitis.
The evidence-based natural history of ulcerative colitis begins with exposure to oxidative stressors (a), which increases colonocyte hydrogen peroxide (H2O2) (b). The increase in colonocyte H2O2 facilitates extracellular diffusion which overwhelms (oxidizes) local interstitial serum albumin antioxidant defense (60% of serum albumin is interstitial), leading to directed migration of neutrophils (chemotaxis) into the colonic epithelium (c) and mucosal inflammation (d) with subsequent development of ulcerative colitis (e). Large amounts of H2O2 are released by neutrophils into the extracellular space (f) with further oxidation of interstitial albumin and exhaustion of tissue antioxidant capacity (c). This worsens colonic inflammation (d) leading to local and systemic reductive depletion (i) as albumin is circulated through the colonic interstitium into tissue lymphatics and back into the systemic circulation. Neutrophil released H2O2 “back flows” into colonocytes (g) adding to the already elevated intracellular H2O2 levels resulting in mitochondrial DNA damage and mitochondrial heteroplasmy (b, red mtH). Mitochondrial heteroplasmy introduces mutations into the electron transport chain protein subunits, which generate additional H2O2 via enhanced electron leakage setting up a vicious cycle of ever increasing colonocyte H2O2 (b, red arrows). Increased colonocyte H2O2 diffuses into the extracellular space (h) causing disease relapse (c, d, e). The combination of local and systemic reductive depletion along with a ready supply of H2O2 from colonocytes and neutrophils (b and d) creates a mucosal inflammation that is self-amplifying, forward propagating, and auto-initiating (relapsing). Elimination of neutrophilic inflammation (d) by any means (i.e., immunosuppressive agents) will not stop relapse from occurring as colonocyte H2O2 continues to diffuse into the extracellular space (c, h). Conversely, normalizing colonocyte H2O2 alone will not stop the inflammation, which has become self-sustaining. This indicates that simultaneous elimination of all pathological sources contributing H2O2 to the inflammatory field must be achieved to ensure long-term remission and normal colonic functionality. Systemic reductive depletion may contribute to other serious health hazards as detailed below. H2O2: Hydrogen peroxide.
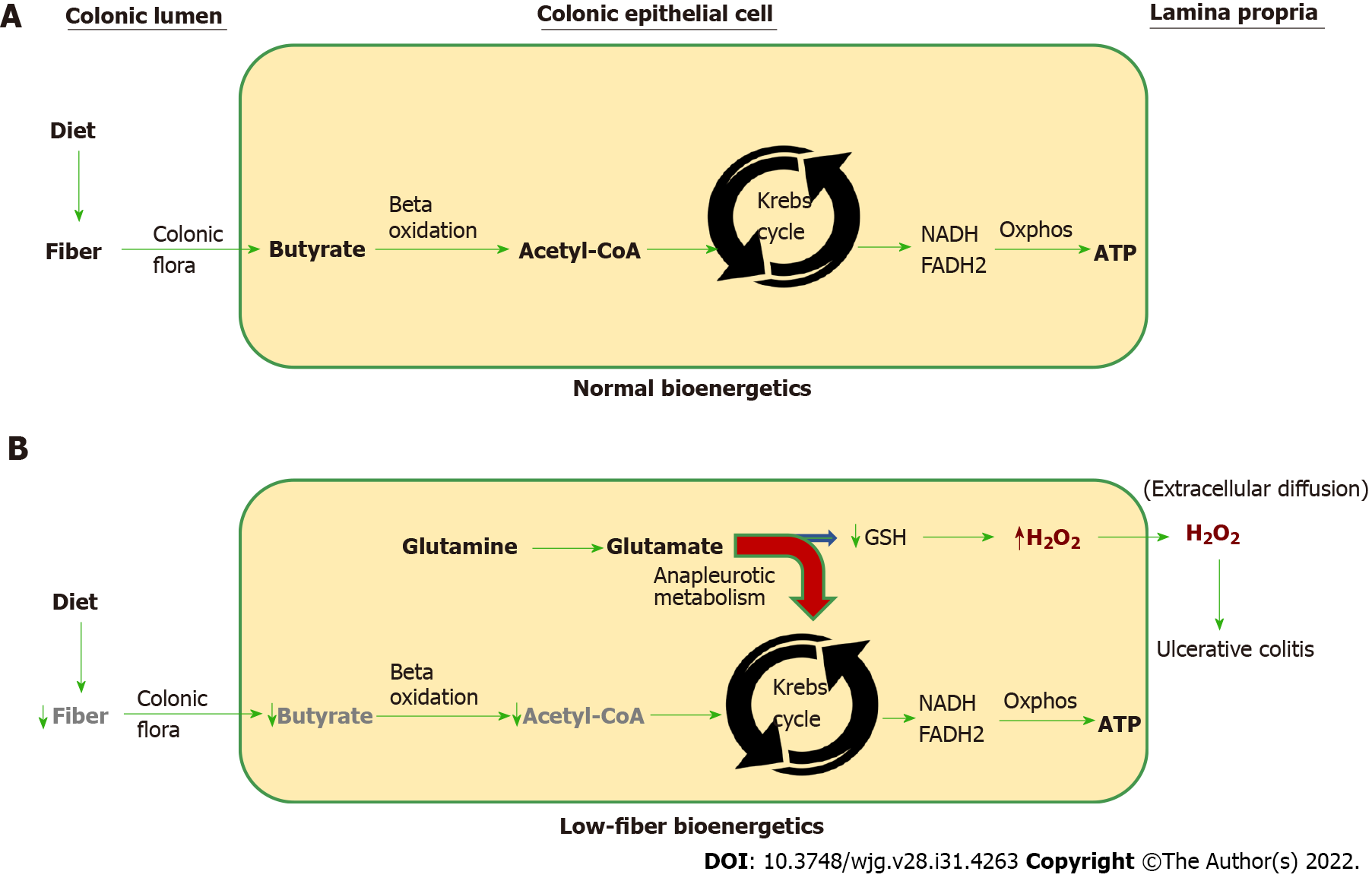
Figure 4 Normal vs low-fiber colonocyte bioenergetics.
A: The normal vectorial bioenergetic flux beginning with soluble dietary fiber that is converted to short-chain fatty acids (i.e., butyrate) by bacteria in the colonic lumen. Butyrate is rapidly absorbed by colonic epithelial cells (colonocytes). Once inside the colonocyte, butyrate undergoes mitochondrial beta-oxidation to generate acetyl-coenzyme A (CoA), which is processed by the Krebs cycle that produces NADH and FADH2. The high-energy electrons present in NADH and FADH2 are used to drive oxidative phosphorylation (Oxphos) resulting in the biosynthesis of ATP, which fuels most of the cell’s energy needs; B: Low fiber intake decreases available butyrate needed for acetyl-CoA production. Under these energy-restricted conditions, glutamate is diverted into the Krebs cycle and away from the synthesis of glutathione (GSH). Diversion of glutamate into the Krebs cycle is called anapleurotic metabolism (red curved arrow) and is needed to replenish depleted Krebs cycle intermediary metabolites that would otherwise be supplied by dietary soluble fiber, which can no longer perform this role due to a low fiber diet. Since glutamate is needed for the synthesis of GSH, the sequestration of glutamate as a replacement energy source restricts the amount of glutathione the cell is able to synthesize. GSH is the principal reducing equivalent required to neutralize cellular hydrogen peroxide (H2O2). Insufficient glutathione will cause cellular H2O2 to increase, which upon extracellular diffusion may initiate neutrophil chemotaxis into the colonic epithelium and de novo ulcerative colitis or disease relapse. Interruption of colonocyte bioenergetic flux anywhere along the pathway from the microbiome to acetyl CoA will increase colonocyte anapleurotic metabolism and cellular H2O2, which can lead to ulcerative colitis. H2O2: Hydrogen peroxide; CoA: Coenzyme A.
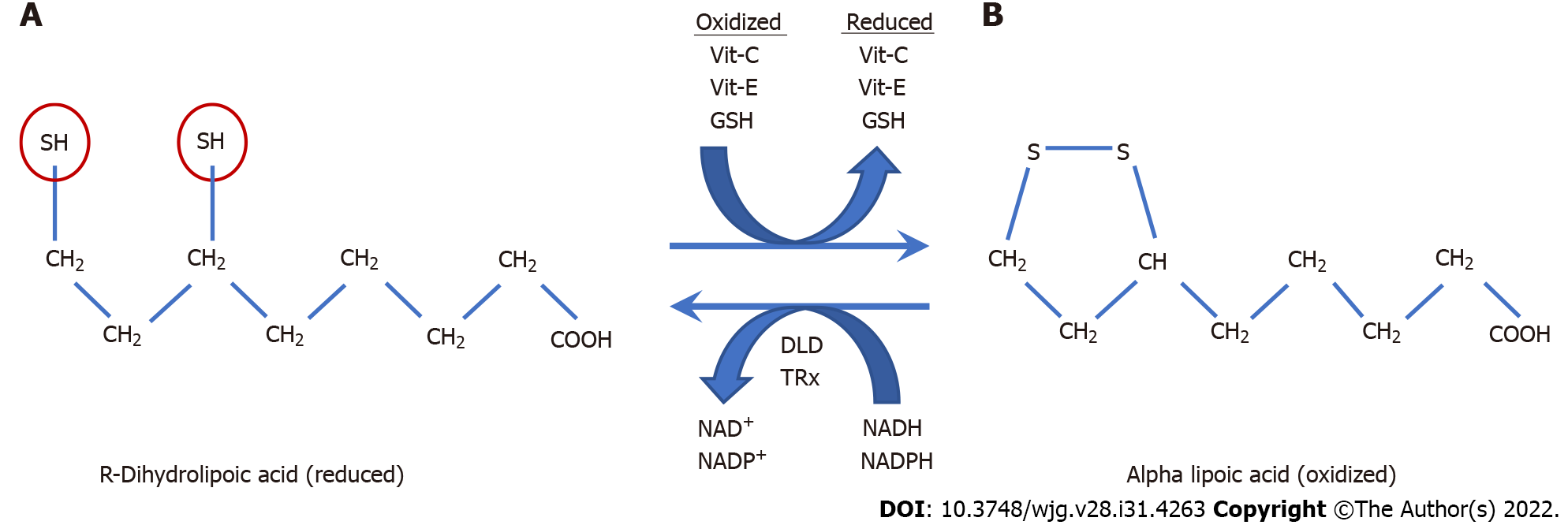
Figure 5 R-dihydrolipoic acid.
A: R-dihydrolipoic acid (RDLA) is the reduced form of alpha lipoic acid (the oxidized form); B: The reducing equivalents of RDLA are provided by its two thiol groups (red circles) that are each capable of donating one electron. RDLA has a redox potential of -290 millivolts which is only exceeded by NADH and NADPH with a redox potential of -320 and -400 millivolts respectively[258]. Due to its very low (more negative) redox potential, RDLA can directly or indirectly reduce all other cellular antioxidants and many types of oxygen radicals[256]. These include vitamin-C, vitamin-E, glutathione, thioredoxin, glutaredoxin, catalase, glutathione peroxidase, and peroxiredoxin[258-261]. Alpha-lipoic acid is reduced by dehydrolipoamide dehydrogenase and Thioredoxin reductase, which use NADH and NADPH as reducing co-factors respectively[262]. The amphipathic nature of RDLA (lipid and water-soluble) allows it to diffuse throughout cellular compartments to transport reducing equivalents where needed and assist in neutralizing excess hydrogen peroxide. The elimination of excess intracellular cellular hydrogen peroxide is essential in order to restore cellular redox homeostasis and prevent ulcerative colitis relapse caused by extracellular diffusion of colonocyte hydrogen peroxide. Vit: Vitamin; DLD: Dehydrolipoamide dehydrogenase; TRx: Thioredoxin reductase; GSH: Glutathione.
- Citation: Pravda J. Evidence-based pathogenesis and treatment of ulcerative colitis: A causal role for colonic epithelial hydrogen peroxide. World J Gastroenterol 2022; 28(31): 4263-4298
- URL: https://www.wjgnet.com/1007-9327/full/v28/i31/4263.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i31.4263