Copyright
©The Author(s) 2022.
World J Gastroenterol. Jul 28, 2022; 28(28): 3535-3554
Published online Jul 28, 2022. doi: 10.3748/wjg.v28.i28.3535
Published online Jul 28, 2022. doi: 10.3748/wjg.v28.i28.3535
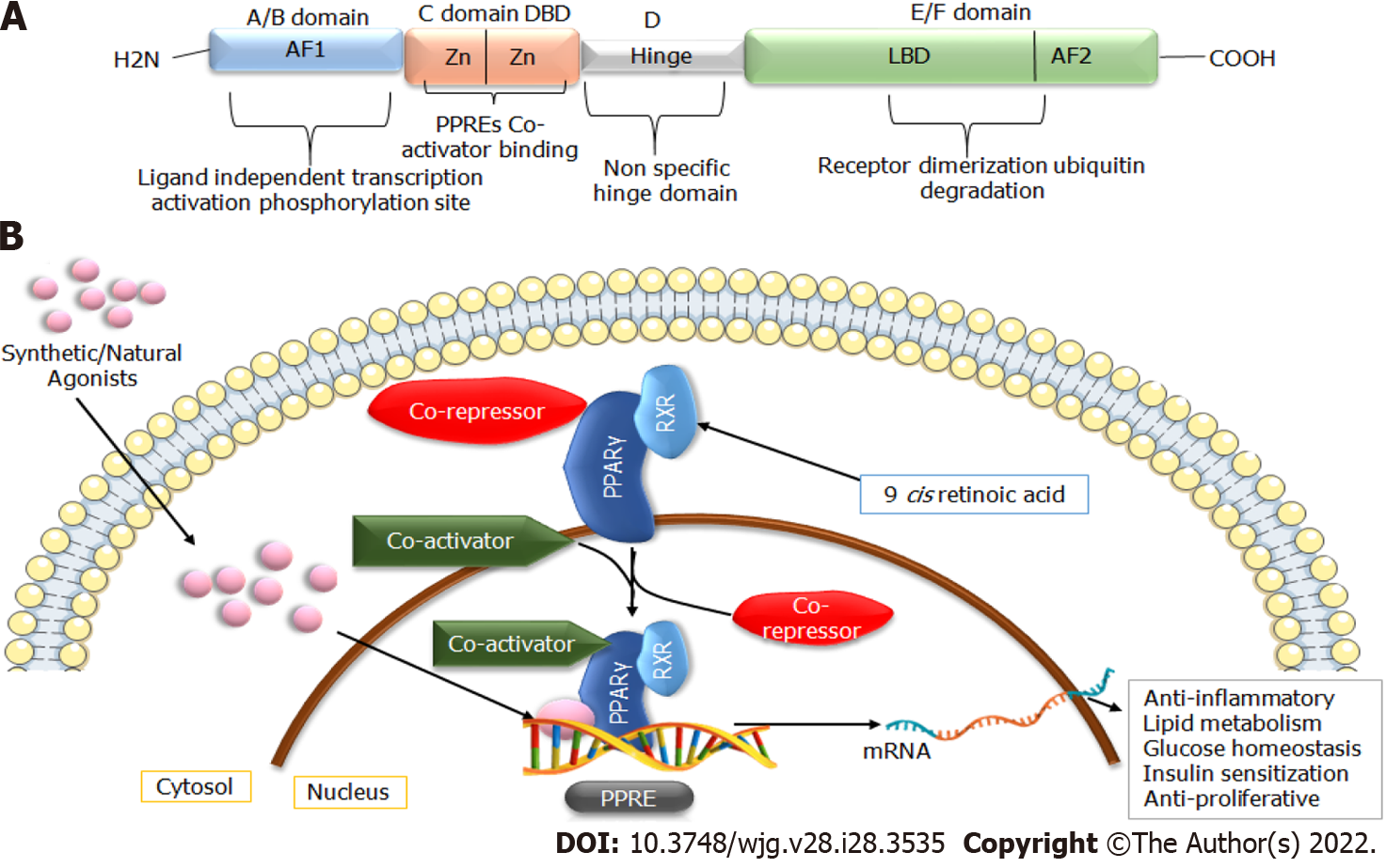
Figure 1 General structure and ligand-activated transcription of peroxisome proliferator-activated receptor-gamma.
A: Peroxisome proliferator-activated receptor (PPAR) structure includes four distinct structural domains A/B, C, D, and E/F; B: Ligand-activated transcription of PPARγ, which includes heterodimerization with nuclear receptor retinoid X receptor (RXR) and binding with peroxisome proliferator response elements located in the target genes through the DNA-binding domain (DBD). In the absence of ligand, PPAR is linked with the corepressor complex, whereas, in the presence of ligand, it is associated with the coactivator complex. LBD: Ligand-binding domain; PPRE: Peroxisome proliferator response element.
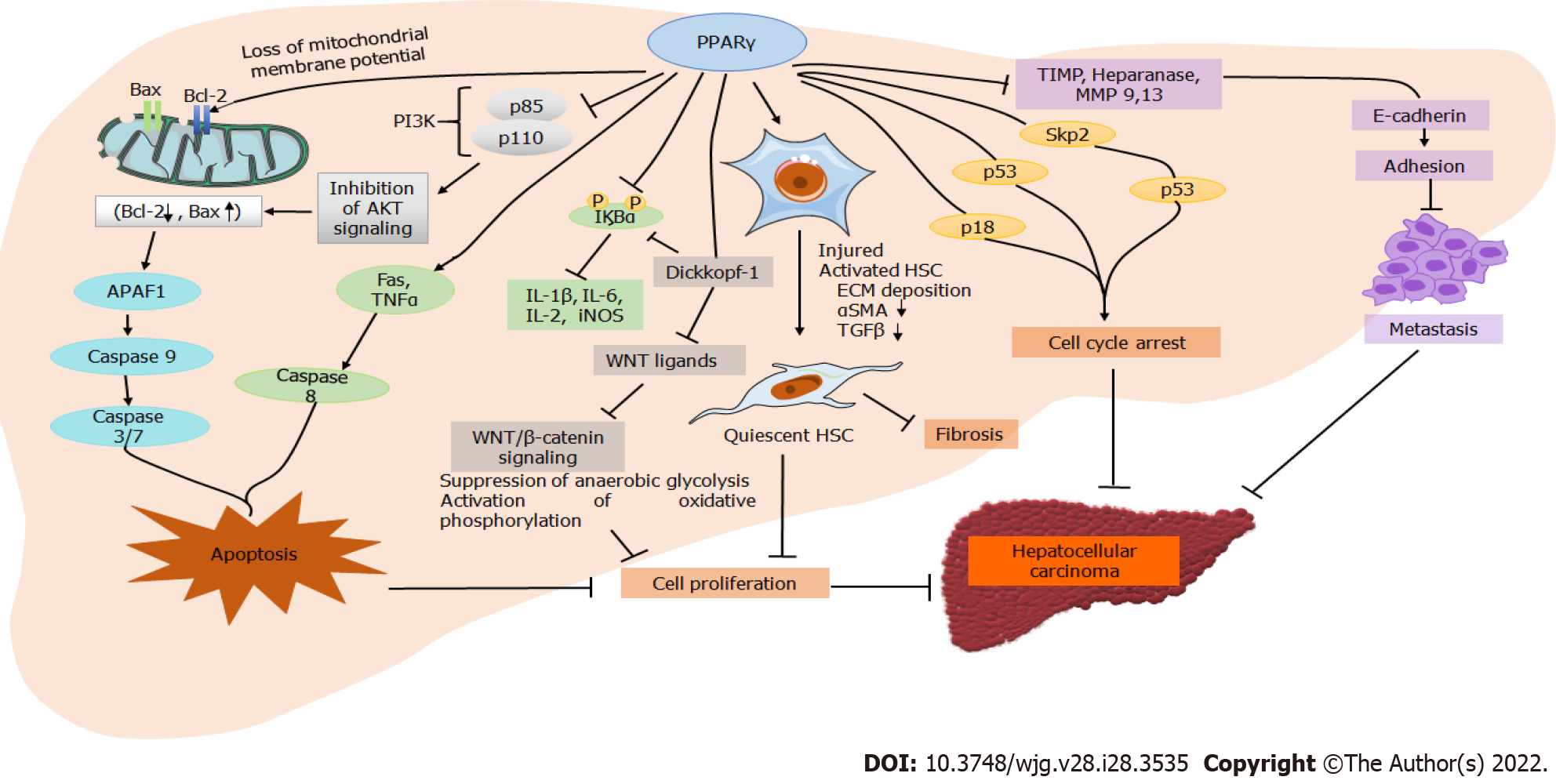
Figure 2 Schematic diagram showing the protective effect of peroxisome proliferator-activated receptor γ against the progression of hepatocellular carcinoma.
Activated peroxisome proliferator-activated receptor γ (PPARγ) interacts with multiple pathways, leading to cell cycle arrest, apoptosis, inhibition of cell proliferation, and cell metastasis in hepatocellular carcinoma. BAX: B-cell lymphoma 2 (Bcl-2)-associated X protein; IκBα: Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibition alpha; IL: Interleukin; TIMP: Tissue inhibitor of metalloproteinases; MMP: Matrix metalloproteinase; ECM: Extracellular matrix; αSMA: Alpha-smooth muscle actin; TGFβ: Transforming growth factor beta; iNOS: Inducible nitric oxide synthase; TNFα: Tumor necrosis factor alpha; APF1: Apoptotic protease activating factor 1.
- Citation: Katoch S, Sharma V, Patial V. Peroxisome proliferator-activated receptor gamma as a therapeutic target for hepatocellular carcinoma: Experimental and clinical scenarios. World J Gastroenterol 2022; 28(28): 3535-3554
- URL: https://www.wjgnet.com/1007-9327/full/v28/i28/3535.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i28.3535