Copyright
©The Author(s) 2022.
World J Gastroenterol. Mar 21, 2022; 28(11): 1172-1183
Published online Mar 21, 2022. doi: 10.3748/wjg.v28.i11.1172
Published online Mar 21, 2022. doi: 10.3748/wjg.v28.i11.1172
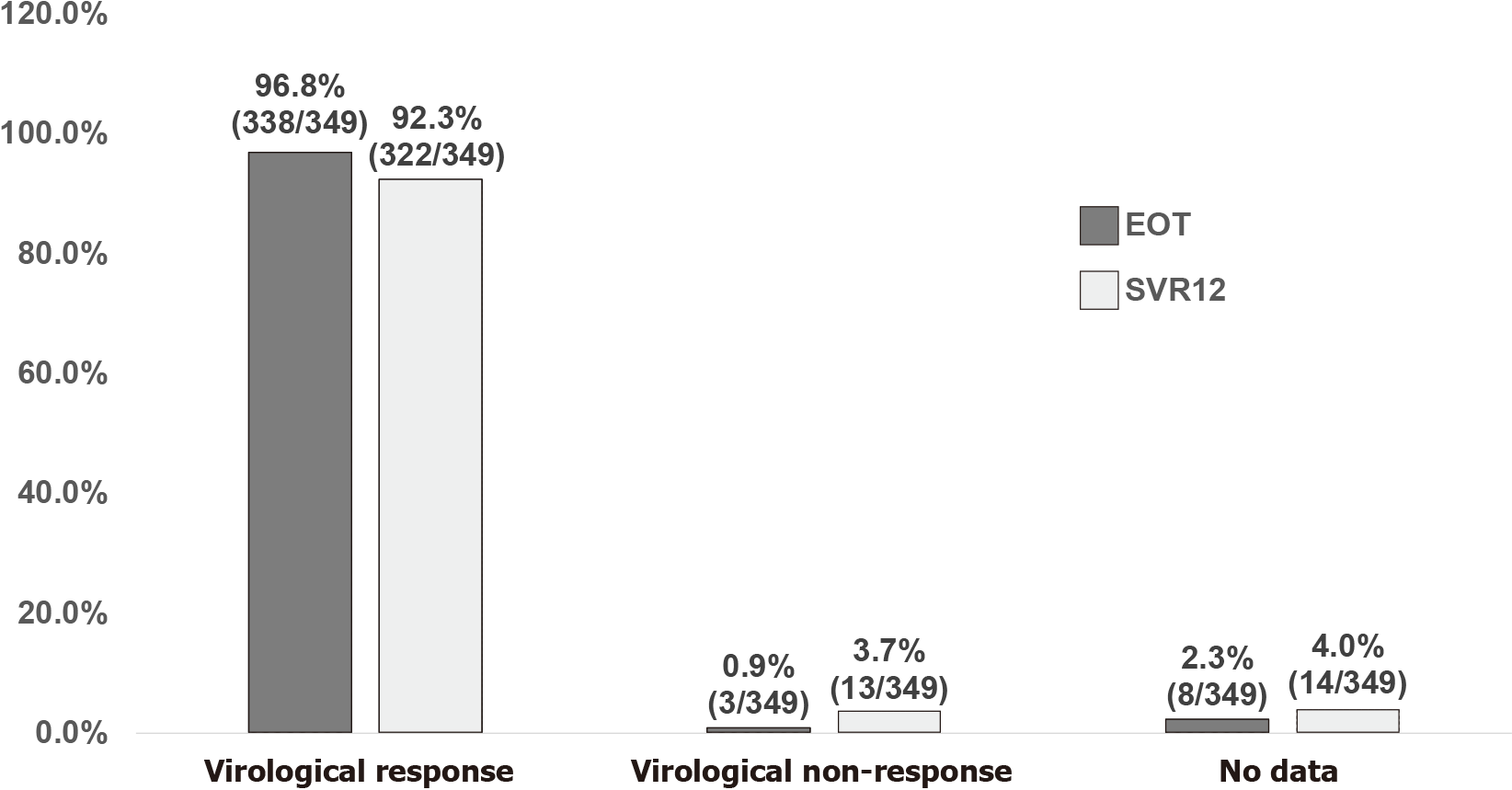
Figure 1 Overall virologic responses at end of treatment and sustained virologic response 12 wk off-therapy.
EOT: End of treatment; SVR12: Sustained virologic response 12 wk off-therapy.
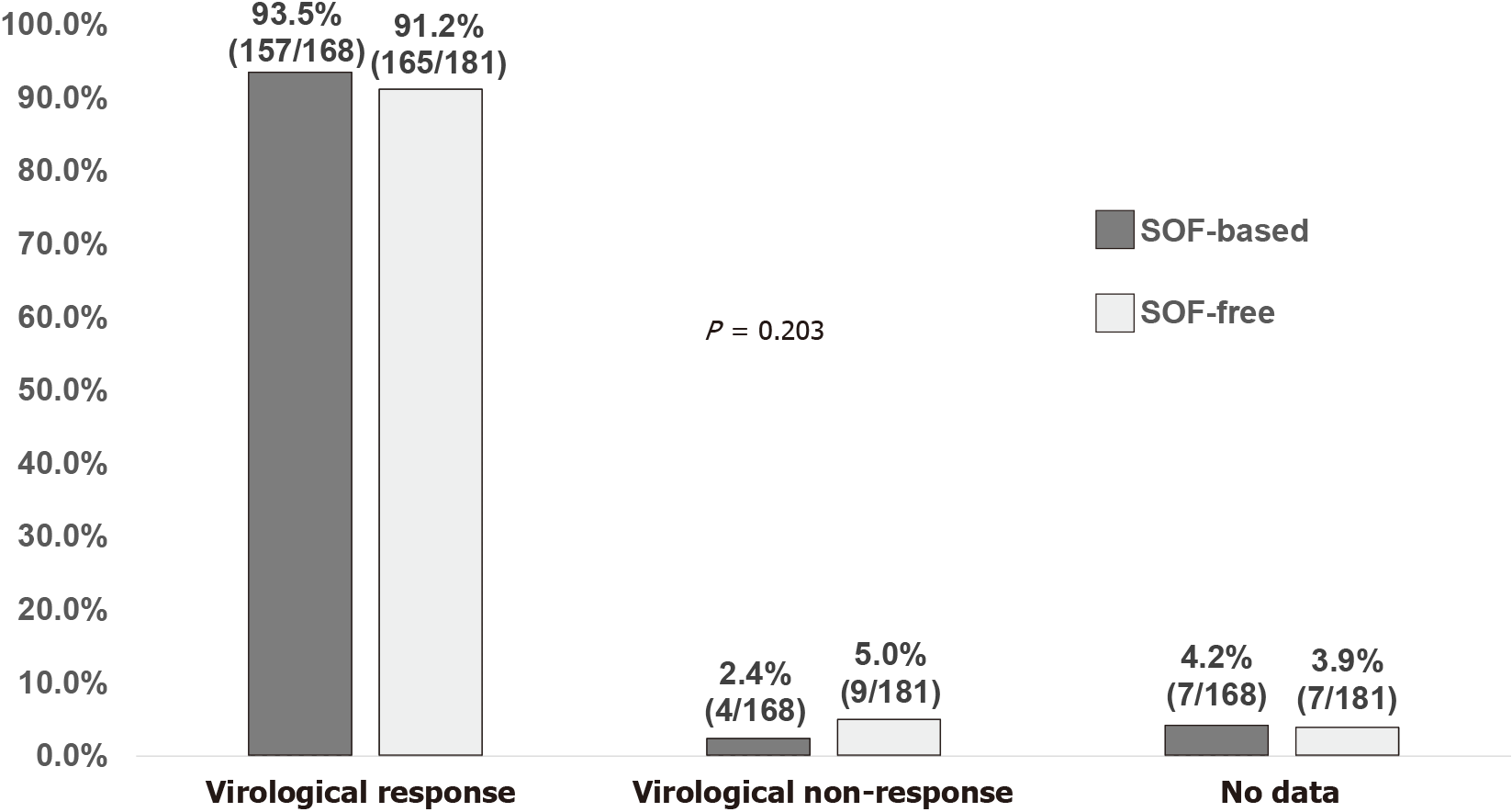
Figure 2 Sustained virologic response 12 wk off-therapy stratified by sofosbuvir-based or sofosbuvir-free regimens.
SOF: Sofosbuvir.
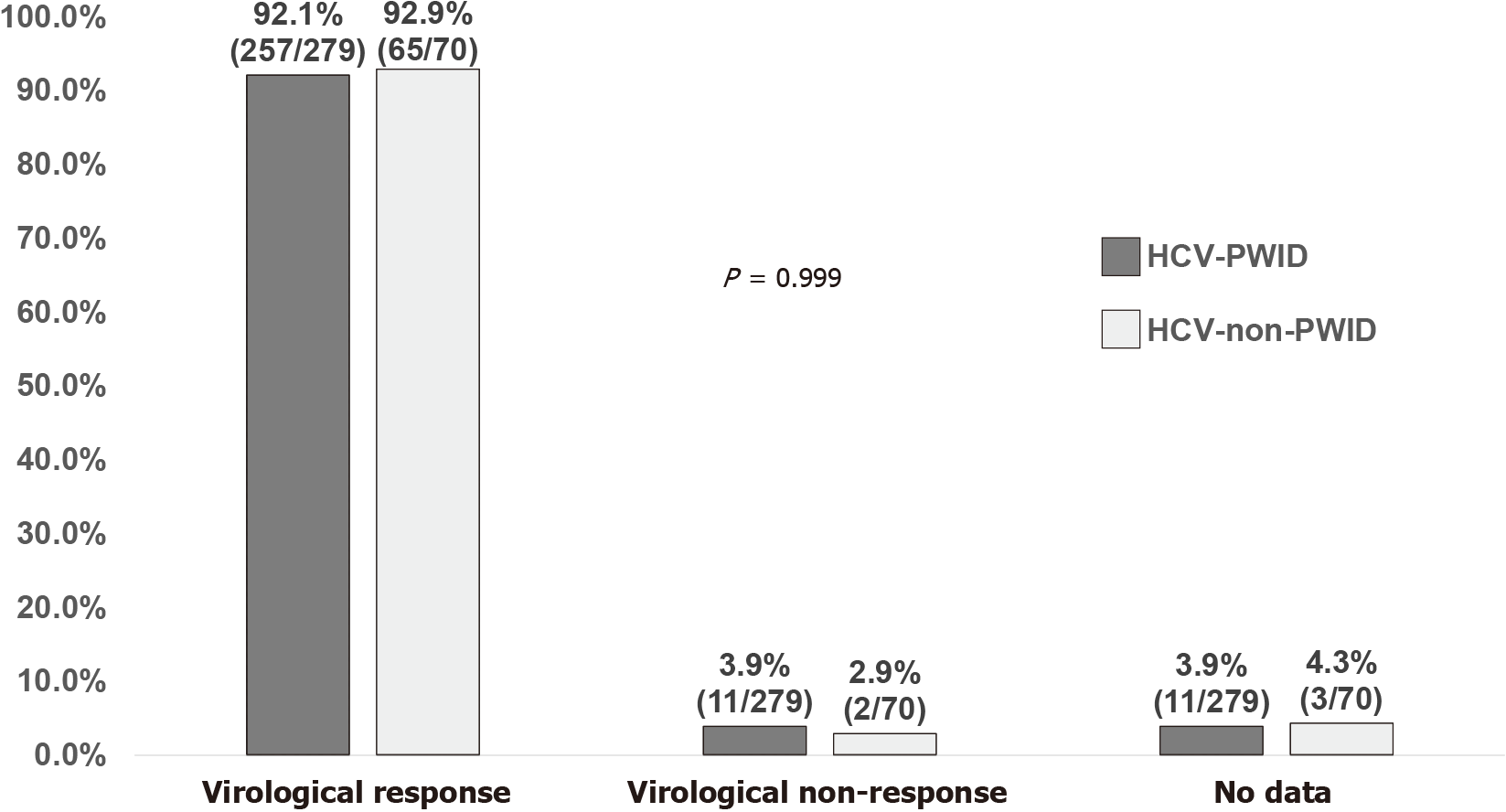
Figure 3 Sustained virologic response at sustained virologic response 12 wk off-therapy stratified by transmission risk of hepatitis C virus infection.
HCV: Hepatitis C virus; PWID: People who inject drugs; non-PWID: Non-people who inject drugs.
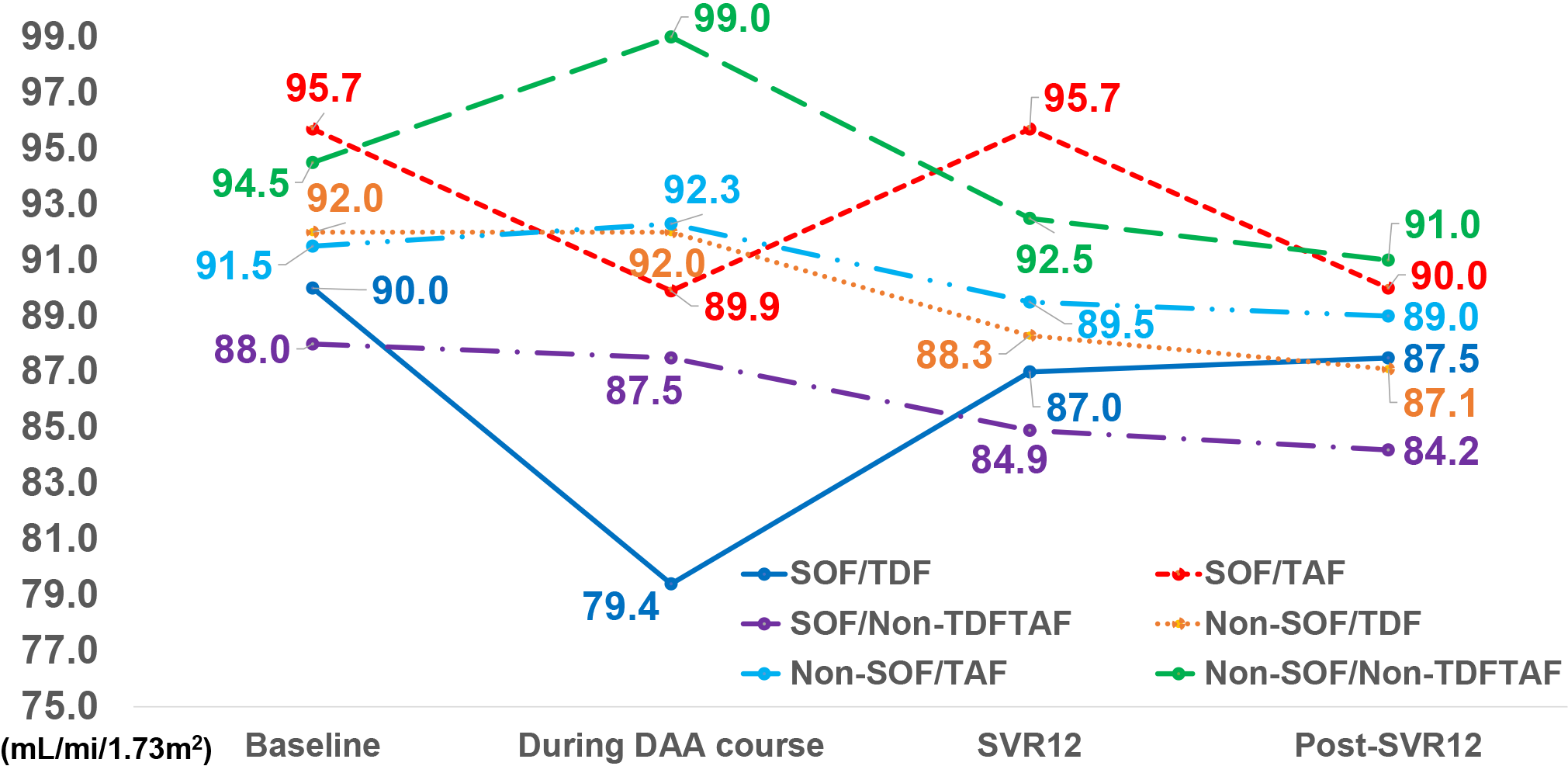
Figure 4 Comparisons of sequential changes of median eGFR from baseline, during DAA course, SVR12, and post-SVR12 stratified by SOF/TDF, SOF/TAF, SOF/non-TDF/non-TAF, non-SOF/TDF, non-SOF/TAF, and non-SOF/non-TDF/non-TAF regimens.
eGFR: Estimated glomerular filtration rate; DAA: Direct-acting antivirals; SVR12: Sustained virologic response 12 wk off-therapy; SOF: Sofosbuvir; TDF: Tenofovir disoproxil fumarate; TAF: Tenofovir alafenamide.
- Citation: Sun HY, Cheng CY, Lin CY, Yang CJ, Lee NY, Liou BH, Tang HJ, Liu YM, Lee CY, Chen TC, Huang YC, Lee YT, Tsai MJ, Lu PL, Tsai HC, Wang NC, Hung TC, Cheng SH, Hung CC. Real-world effectiveness of direct-acting antivirals in people living with human immunodeficiency virus and hepatitis C virus genotype 6 infections. World J Gastroenterol 2022; 28(11): 1172-1183
- URL: https://www.wjgnet.com/1007-9327/full/v28/i11/1172.htm
- DOI: https://dx.doi.org/10.3748/wjg.v28.i11.1172