Copyright
©The Author(s) 2021.
World J Gastroenterol. Nov 7, 2021; 27(41): 7190-7206
Published online Nov 7, 2021. doi: 10.3748/wjg.v27.i41.7190
Published online Nov 7, 2021. doi: 10.3748/wjg.v27.i41.7190
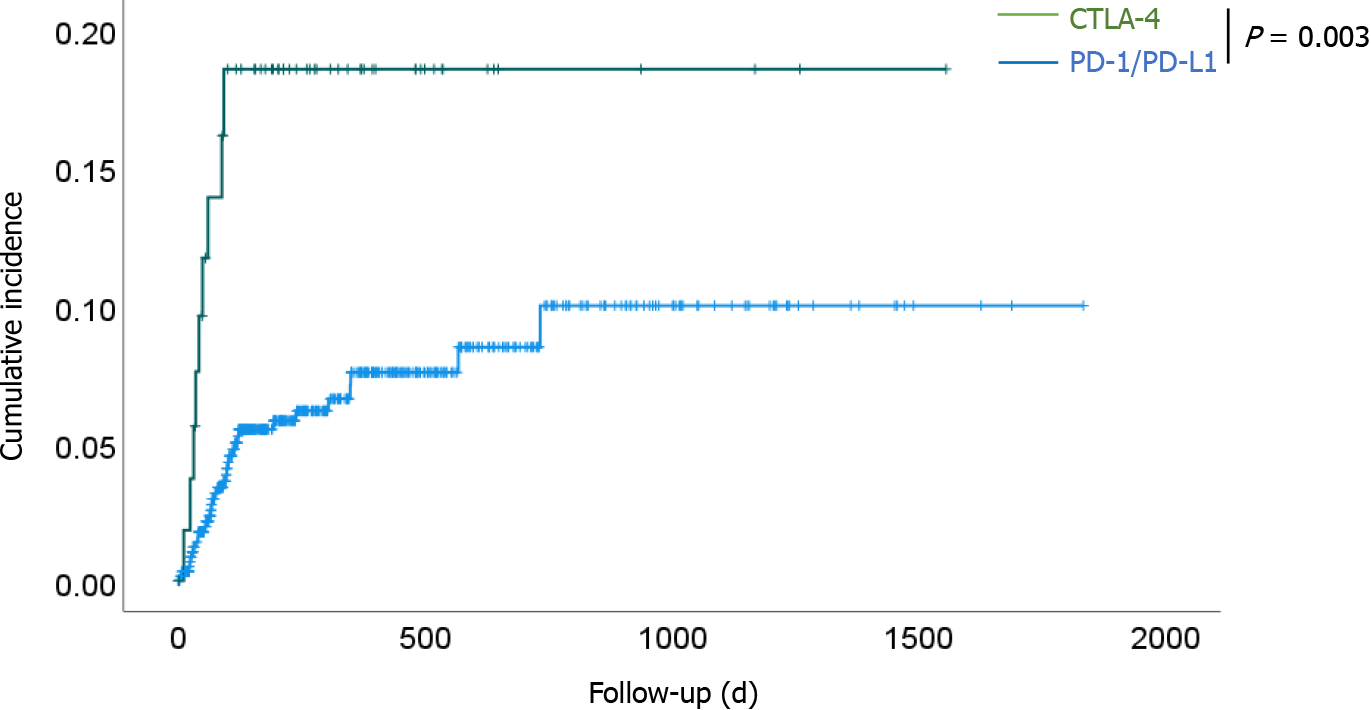
Figure 1 Kaplan–Meier curves of the cumulative incidence of gastrointestinal- immune-related adverse events for all patients in the programmed cell death-1/programmed death-ligand 1 and cytotoxic T-lymphocyte antigen 4 groups.
The cumulative incidence was significantly higher in the cytotoxic T-lymphocyte antigen 4 group than in the programmed cell death-1/programmed death-ligand 1 group (P = 0.003). CTLA-4: Cytotoxic T-lymphocyte antigen 4; PD-1: Programmed cell death-1; PD-L1: Programmed death-ligand 1.
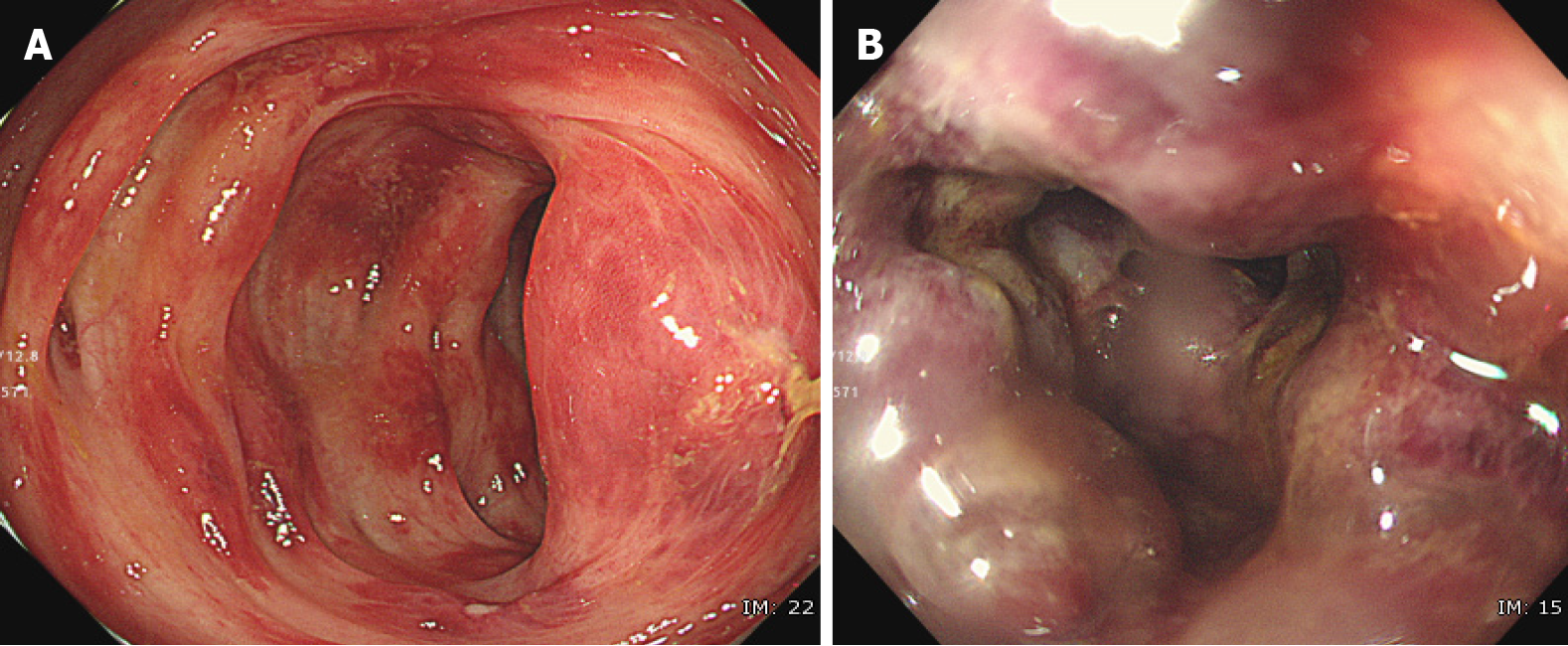
Figure 2 Ischemic change caused by an immune checkpoint inhibitor.
Endoscopic images of gastrointestinal-immune-related adverse events in the sigmoid colon of a patient. A: The mucosa of the sigmoid colon shows redness, erosion, hemorrhage, and edema; B: Some of the mucosa is pale, with submucosal edema and bleeding.
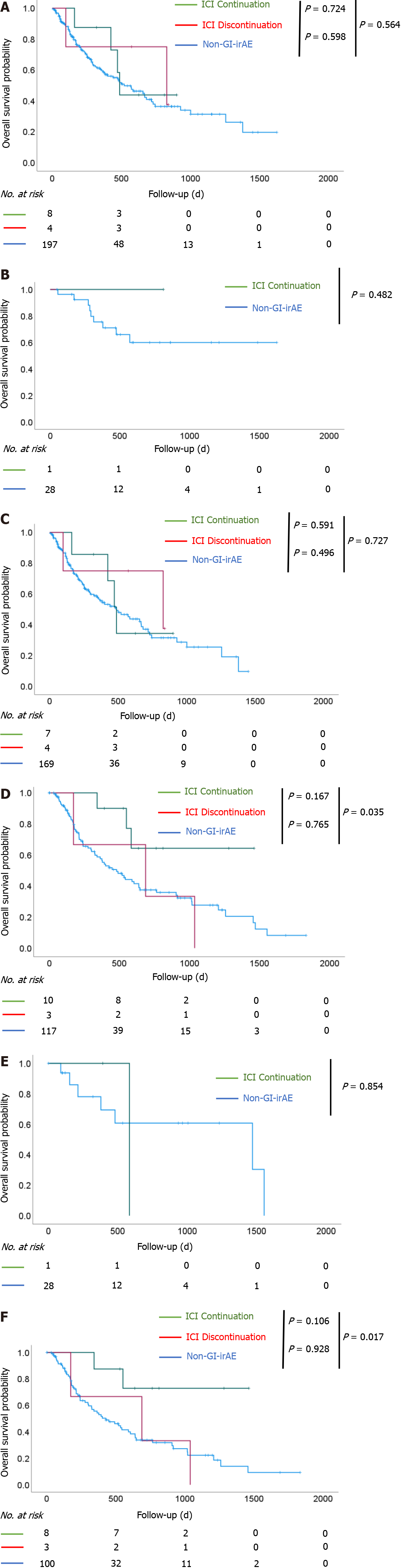
Figure 3 Overall survival after initiation of immune checkpoint inhibitor treatment.
Overall survival (OS) of patients with non-small cell lung cancer (NSCLC) and OS of patients with malignant melanoma (MM). A: In patients with NSCLC, there was no significant difference in OS among the three groups; B: The results were similar when stratified in stage III; C: The results were similar when stratified in stage IV; D: In patients with MM, there was a significant prolongation of OS in the immune checkpoint inhibitor (ICI) continuation group compared to the non-gastrointestinal immune-related adverse event (non-GI-irAE) group (P = 0.035); E: There was no significant difference in OS between the ICI continuation group and the non-GI-irAE group among patients with stage III disease; F: Among patients with stage IV disease, there was a significant prolongation of OS in the ICI continuation group compared to the non-GI-irAE group (P = 0.017). ICI continuation group: Patients who continued ICI treatment after developing GI-irAEs; ICI discontinuation group: Patients who discontinued ICI treatment after developing GI-irAEs; non-GI-irAE group: Patients with no GI-irAEs.
- Citation: Yamada K, Sawada T, Nakamura M, Yamamura T, Maeda K, Ishikawa E, Iida T, Mizutani Y, Kakushima N, Ishikawa T, Furukawa K, Ohno E, Honda T, Kawashima H, Ishigami M, Furune S, Hase T, Yokota K, Maeda O, Hashimoto N, Akiyama M, Ando Y, Fujishiro M. Clinical characteristics of gastrointestinal immune-related adverse events of immune checkpoint inhibitors and their association with survival. World J Gastroenterol 2021; 27(41): 7190-7206
- URL: https://www.wjgnet.com/1007-9327/full/v27/i41/7190.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i41.7190