Copyright
©The Author(s) 2021.
World J Gastroenterol. Aug 14, 2021; 27(30): 5088-5099
Published online Aug 14, 2021. doi: 10.3748/wjg.v27.i30.5088
Published online Aug 14, 2021. doi: 10.3748/wjg.v27.i30.5088
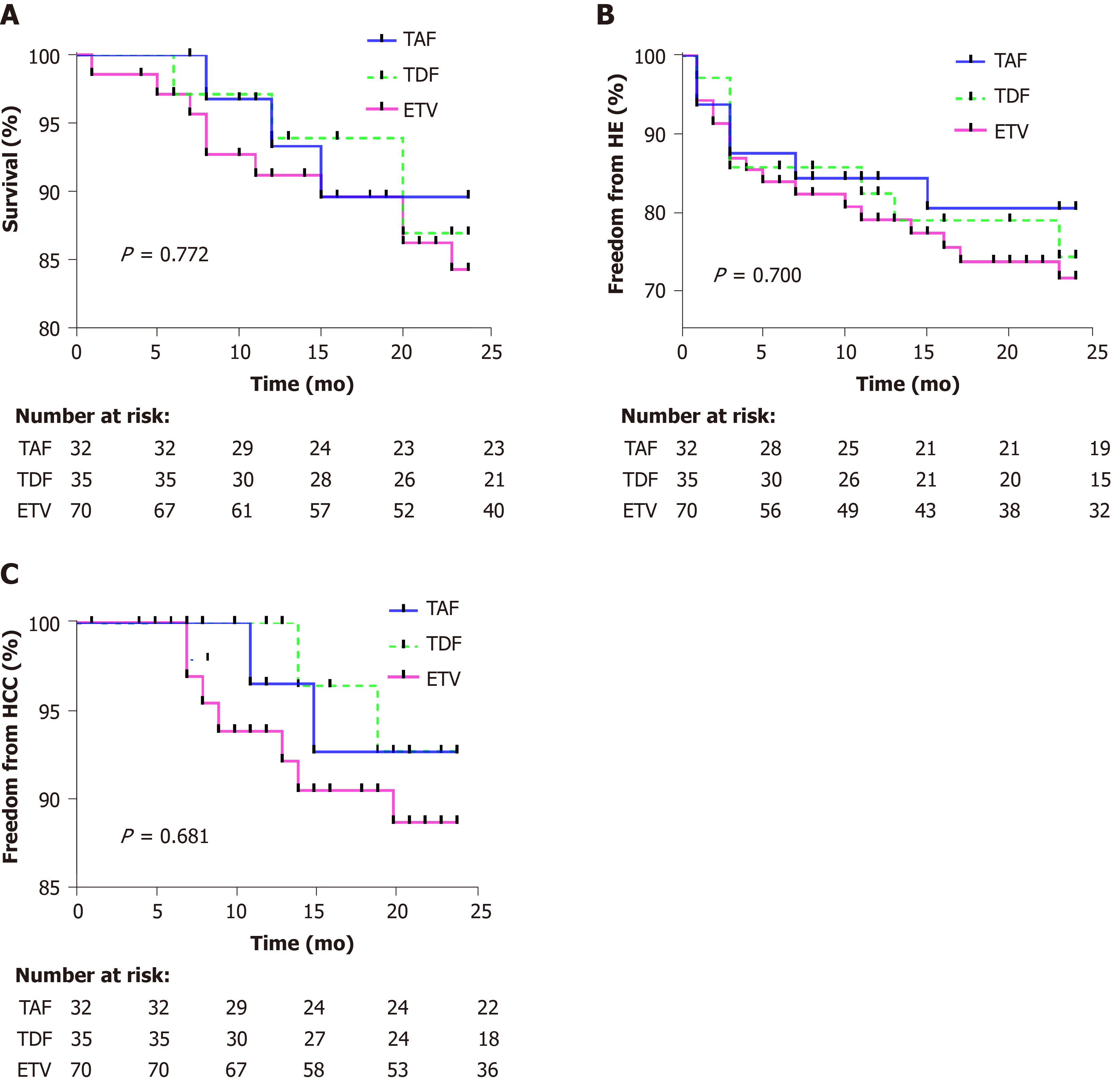
Figure 1 Overall survival, hepatic encephalopathy free survival, and hepatocellular carcinoma free survival rate in tenofovir alafenamide fumarate, tenofovir disoproxil fumarate, and entecavir groups.
A: Survival; B: Freedom from hepatic encephalopathy; C: Freedom from hepatocellular carcinoma. HCC: Hepatocellular carcinoma; HE: Hepatic encephalopathy; TAF: Tenofovir alafenamide fumarate; TDF: Tenofovir disoproxil fumarate; ETV: Entecavir.
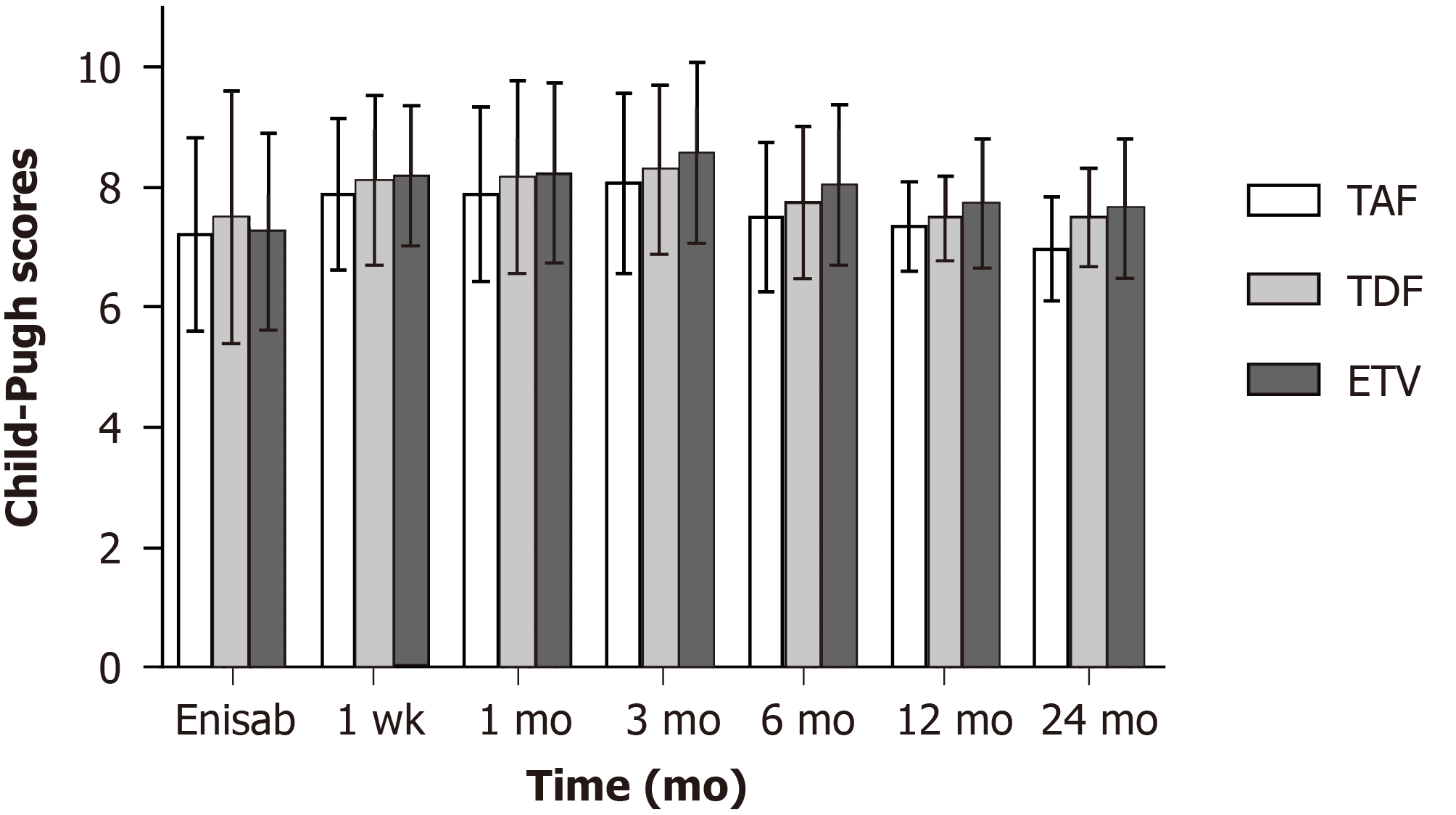
Figure 2 Preoperative and postoperative Child-Pugh scores and ratios in the three patient groups.
TAF: Tenofovir alafenamide fumarate; TDF: Tenofovir disoproxil fumarate; ETV: Entecavir.
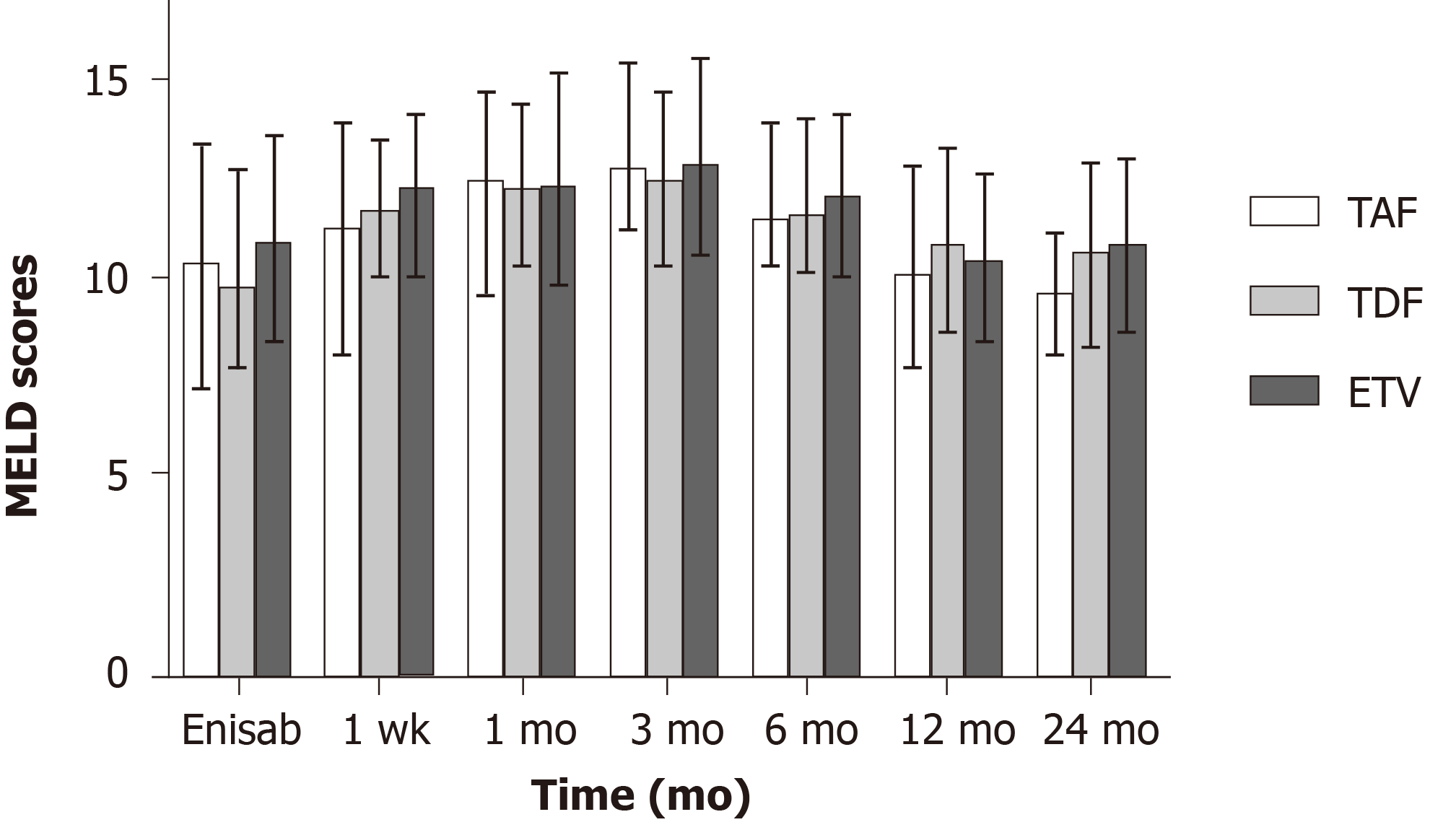
Figure 3 Changes in preoperative and postoperative model for end-stage liver disease scores in the three patient groups.
MELD: Model for end-stage liver disease; TAF: Tenofovir alafenamide fumarate; TDF: Tenofovir disoproxil fumarate; ETV: Entecavir.
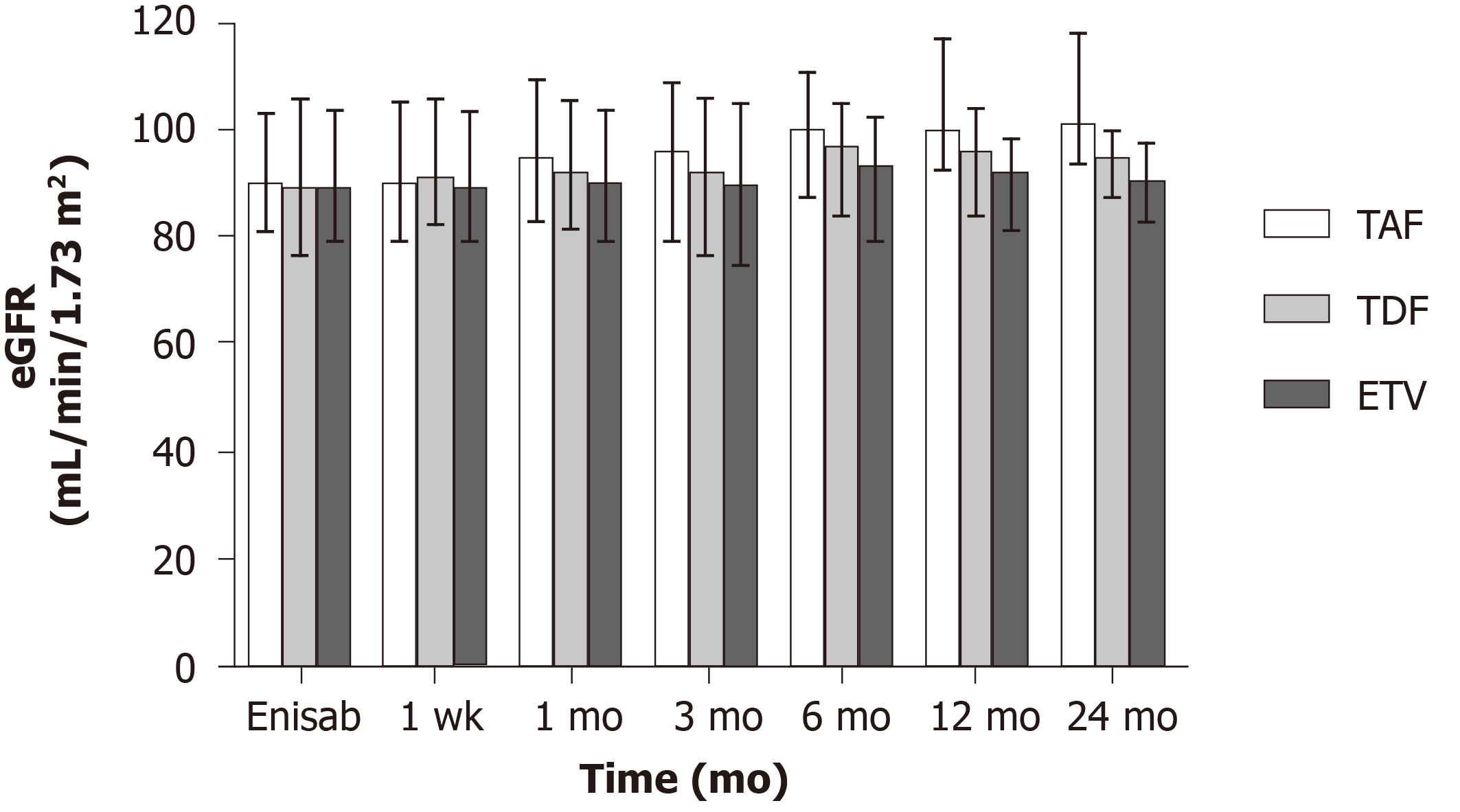
Figure 4 Changes in preoperative and postoperative estimated glomerular filtration rates in the three patient groups.
eGFR: Estimated glomerular filtration rate; TAF: Tenofovir alafenamide fumarate; TDF: Tenofovir disoproxil fumarate; ETV: Entecavir.
- Citation: Yao X, Huang S, Zhou H, Tang SH, Qin JP. Clinical efficacy of antiviral therapy in patients with hepatitis B-related cirrhosis after transjugular intrahepatic portosystemic shunt. World J Gastroenterol 2021; 27(30): 5088-5099
- URL: https://www.wjgnet.com/1007-9327/full/v27/i30/5088.htm
- DOI: https://dx.doi.org/10.3748/wjg.v27.i30.5088