Copyright
©The Author(s) 2020.
World J Gastroenterol. Dec 21, 2020; 26(47): 7497-7512
Published online Dec 21, 2020. doi: 10.3748/wjg.v26.i47.7497
Published online Dec 21, 2020. doi: 10.3748/wjg.v26.i47.7497
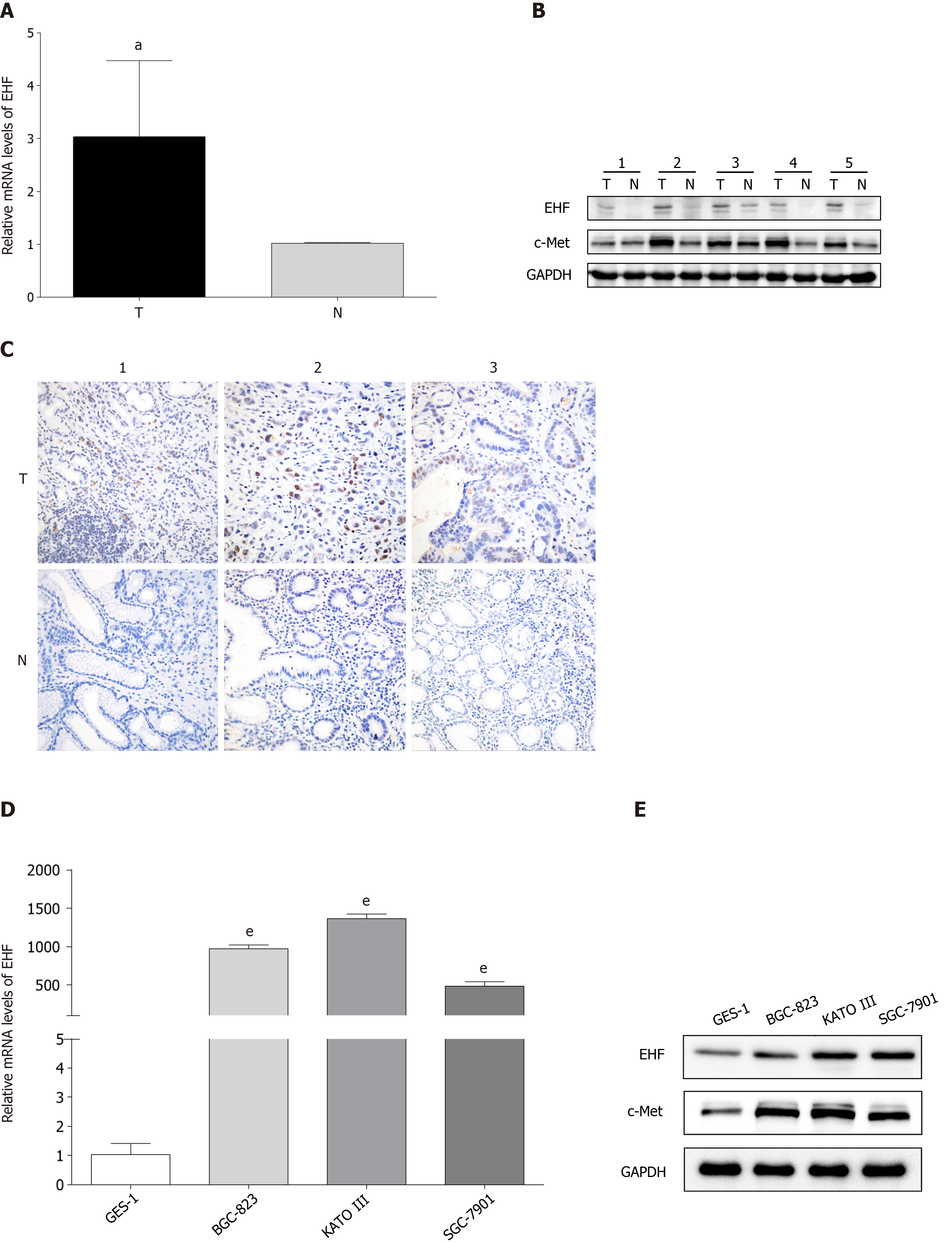
Figure 1 ETS homologous factor and c-Met expression in gastric cancer tissues and cell lines.
A: Quantitative PCR analysis of mRNAs revealed the upregulation of ETS homologous factor (EHF) mRNA in gastric cancer (GC) tissues compared with their matched adjacent tissues; B: Western blotting results showed that the expression of EHF and c-Met were both upregulated in GC tissues; C: Immunohistochemical staining of EHF indicated that increased expression of EHF was higher in GC tissues than that in matched adjacent tissues (400 ×); D: The mRNA expression level of EHF was enhanced in GC cell lines compared with that in normal gastric epithelial cells; E: EHF protein expression was increased in GC cell lines in which c-Met was also upregulated. aP < 0.05 vs matched adjacent tissues; eP < 0.001 vs normal gastric epithelial cells. T: GC tissues; N: Matched adjacent tissues.
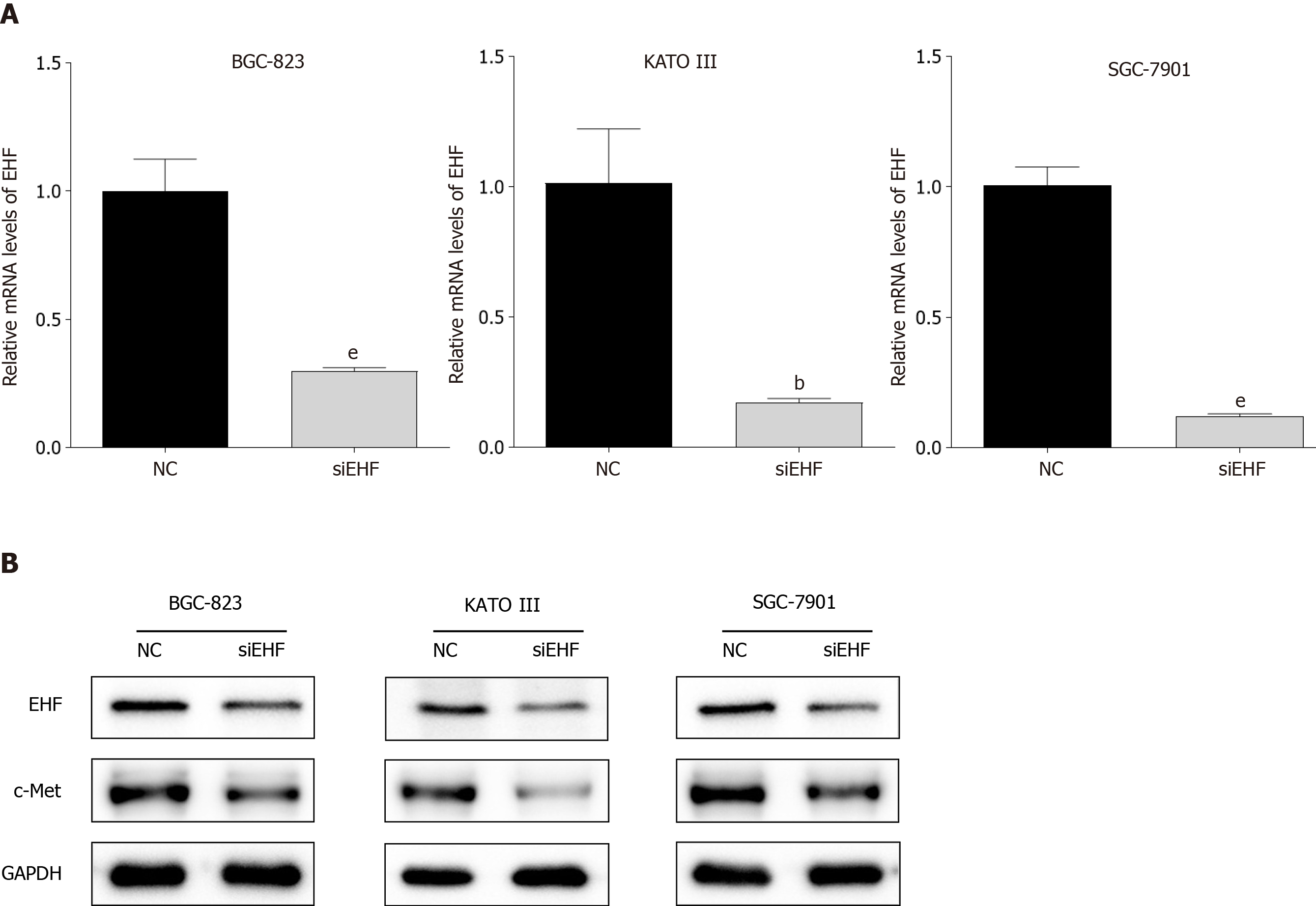
Figure 2 Transfection of ETS homologous factor-targeted small interfering RNA reduces c-Met expression in gastric cancer cell lines.
A: Quantitative PCR was performed to evaluate the mRNA levels of ETS homologous factor (EHF) in gastric cancer (GC) cells following transfection for 72 h; B: Western blotting was used to detect the protein expression of EHF and c-Met after small interfering RNA transfection. bP < 0.01 vs negative control (NC) group; eP < 0.001 vs NC group.
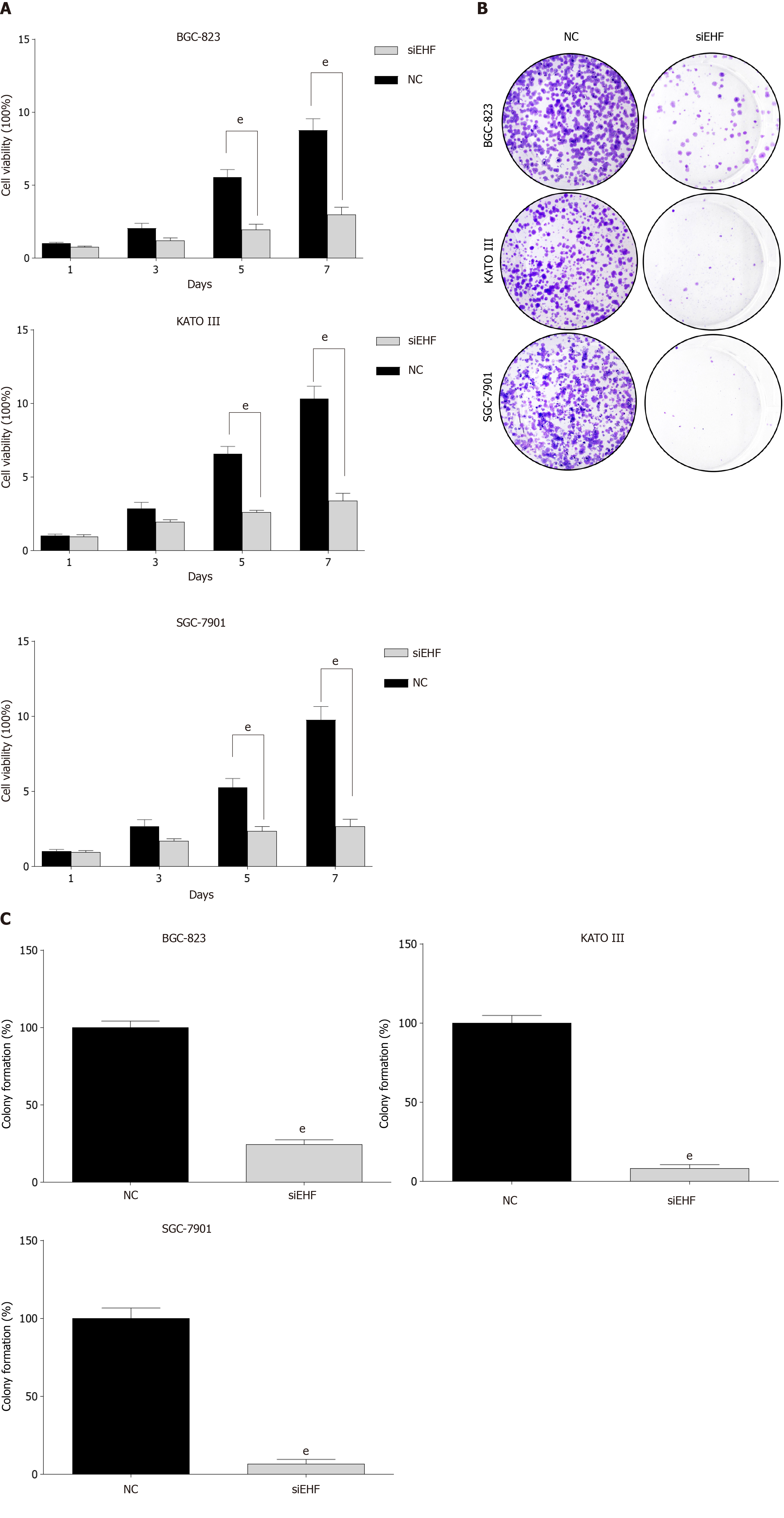
Figure 3 The effects of ETS homologous factor silencing on gastric cancer cell proliferation.
A: Cell viability was evaluated by the Cell Counting Kit-8 after transfection for 1, 3, 5, and 7 d; B and C: Cell colony formation was measured to verify the changes in gastric cancer (GC) cell proliferation following ETS homologous factor downregulation. eP < 0.001 vs negative control (NC) group.
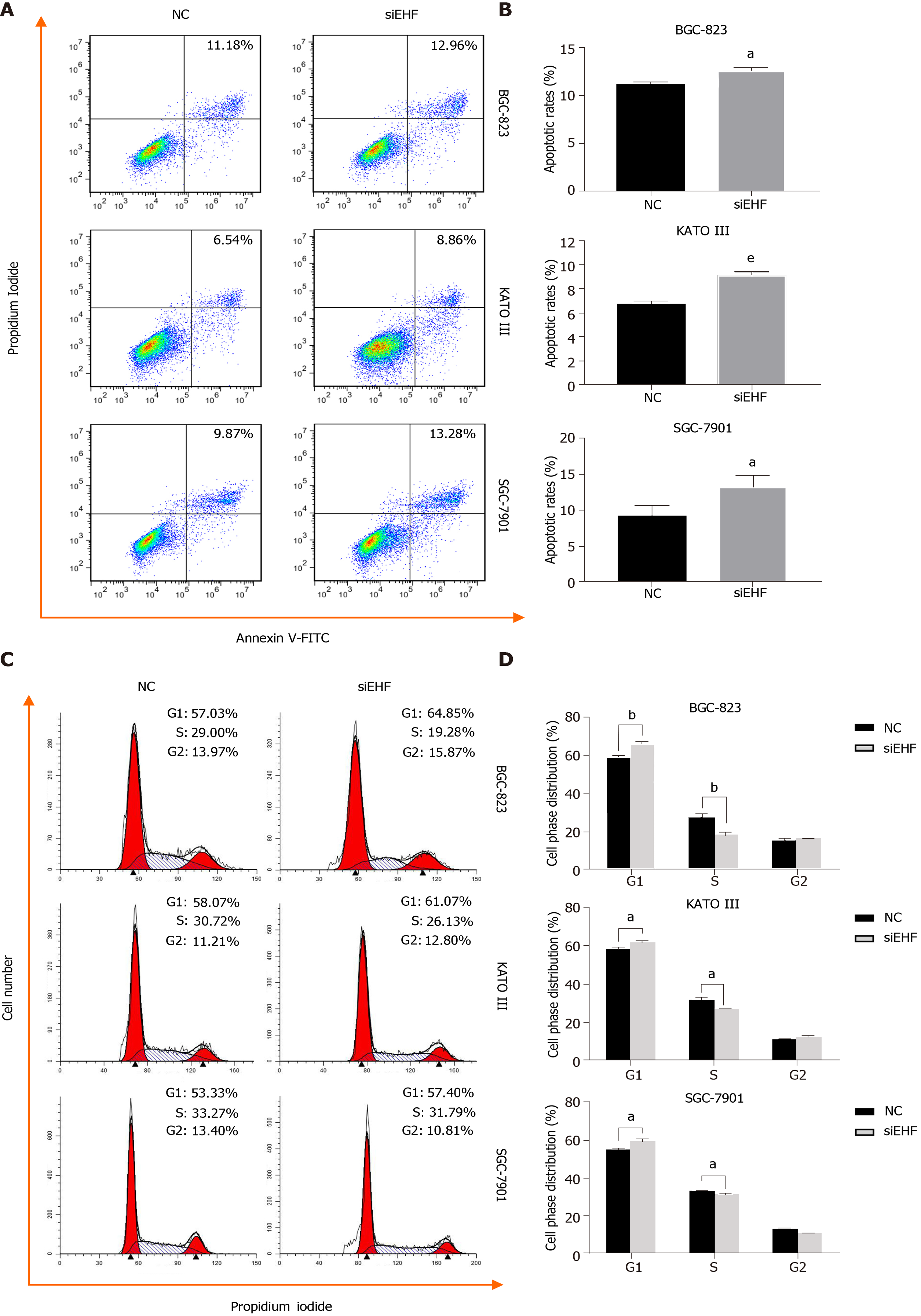
Figure 4 Downregulation of ETS homologous factor induces apoptosis and cell cycle arrest in gastric cancer cells.
A and B: The analysis of apoptosis was performed by flow cytometry to detect the induction of apoptosis caused by ETS homologous factor (EHF) silencing following V-FITC/propidium iodide (PI) staining; C and D: The cell cycle arrest induced by EHF downregulation was assessed by PI staining and further analyzed by flow cytometry. aP < 0.05 vs negative control (NC) group; bP < 0.01 vs NC group; eP < 0.001 vs NC group. GC: Gastric cancer.
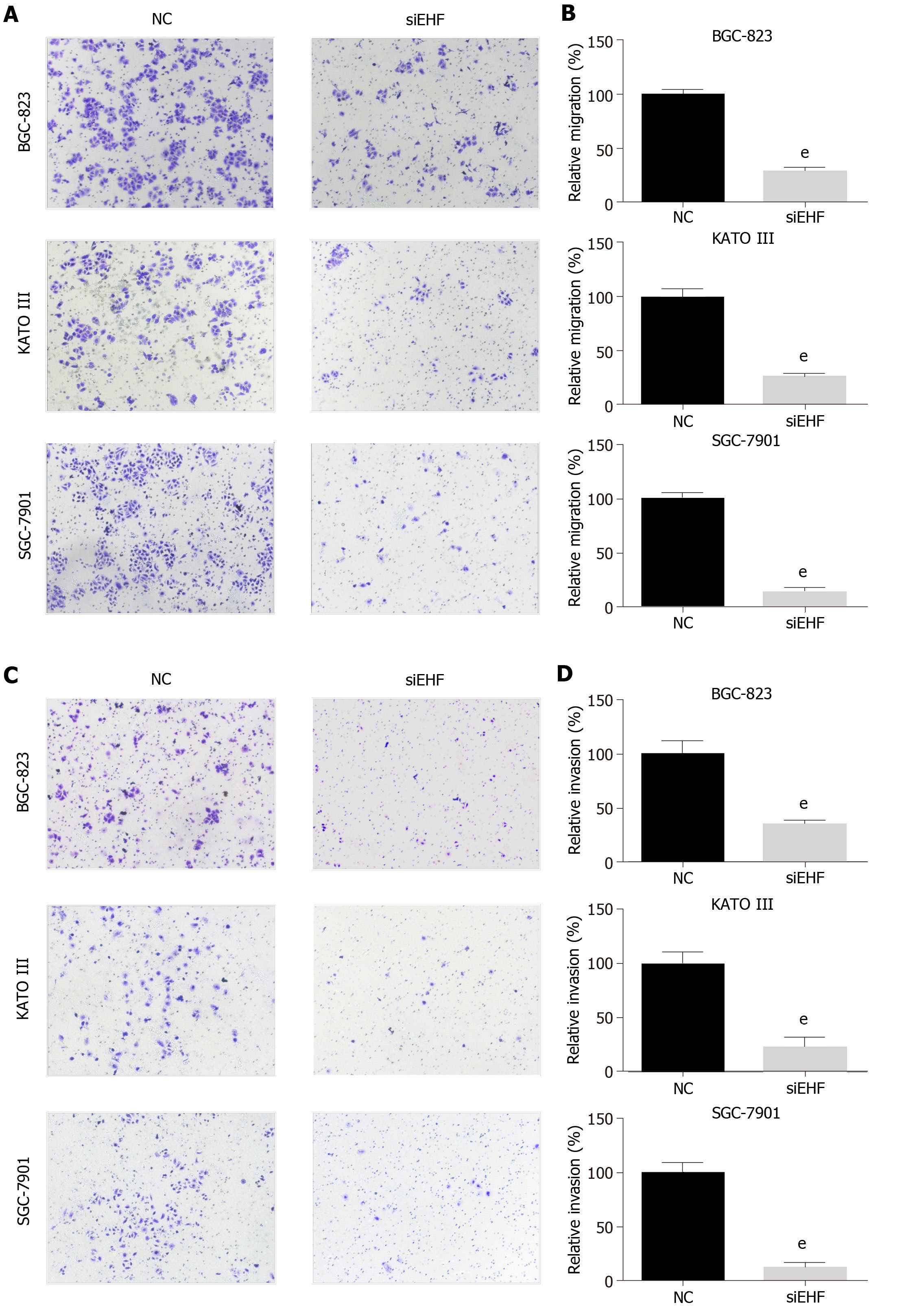
Figure 5 ETS homologous factor regulates the cell migration and invasion abilities of gastric cancer cells.
A and B: The transwell assay was performed to assess the cell migration ability of gastric cancer (GC) cells following ETS homologous factor (EHF) downregulation; C and D: Invasion assays were performed by adding Matrigel into the Transwell chamber to determine the effects of EHF silencing on the invasion ability of GC cells. eP < 0.001 vs negative control (NC) group.
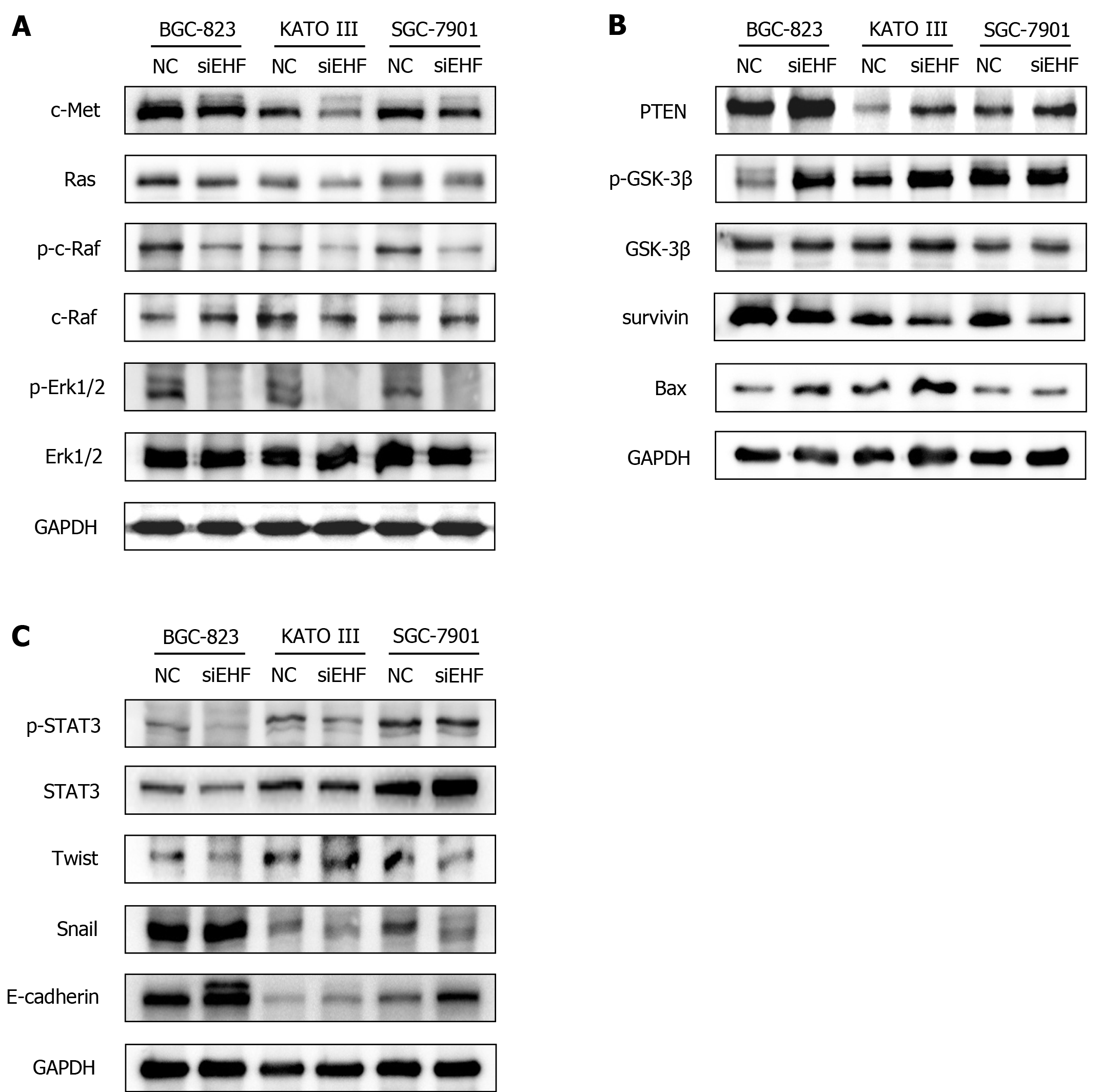
Figure 6 ETS homologous factor promotes the malignant biological behaviors of gastric cancer cells through the c-Met pathway.
Western blotting was conducted to investigate the effects of ETS homologous factor (EHF) silencing on the expression and activities of signaling molecules in the c-Met pathway. A: Alterations in the Ras- extracellular signal-related kinase 1/2 (Erk1/2) cascade after EHF inhibition; B: The molecular changes in phosphatase and tensin homolog (PTEN) and glycogen synthase kinase-3β (GSK3β) and their potential targets following EHF silencing; C: The altered activities of signal transducer and activator of transcription 3 (STAT3) and the changed expression of its downstream effectors after EHF downregulation. GC: Gastric cancer; NC: Negative control group.
- Citation: Gu ML, Zhou XX, Ren MT, Shi KD, Yu MS, Jiao WR, Wang YM, Zhong WX, Ji F. Blockage of ETS homologous factor inhibits the proliferation and invasion of gastric cancer cells through the c-Met pathway. World J Gastroenterol 2020; 26(47): 7497-7512
- URL: https://www.wjgnet.com/1007-9327/full/v26/i47/7497.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i47.7497