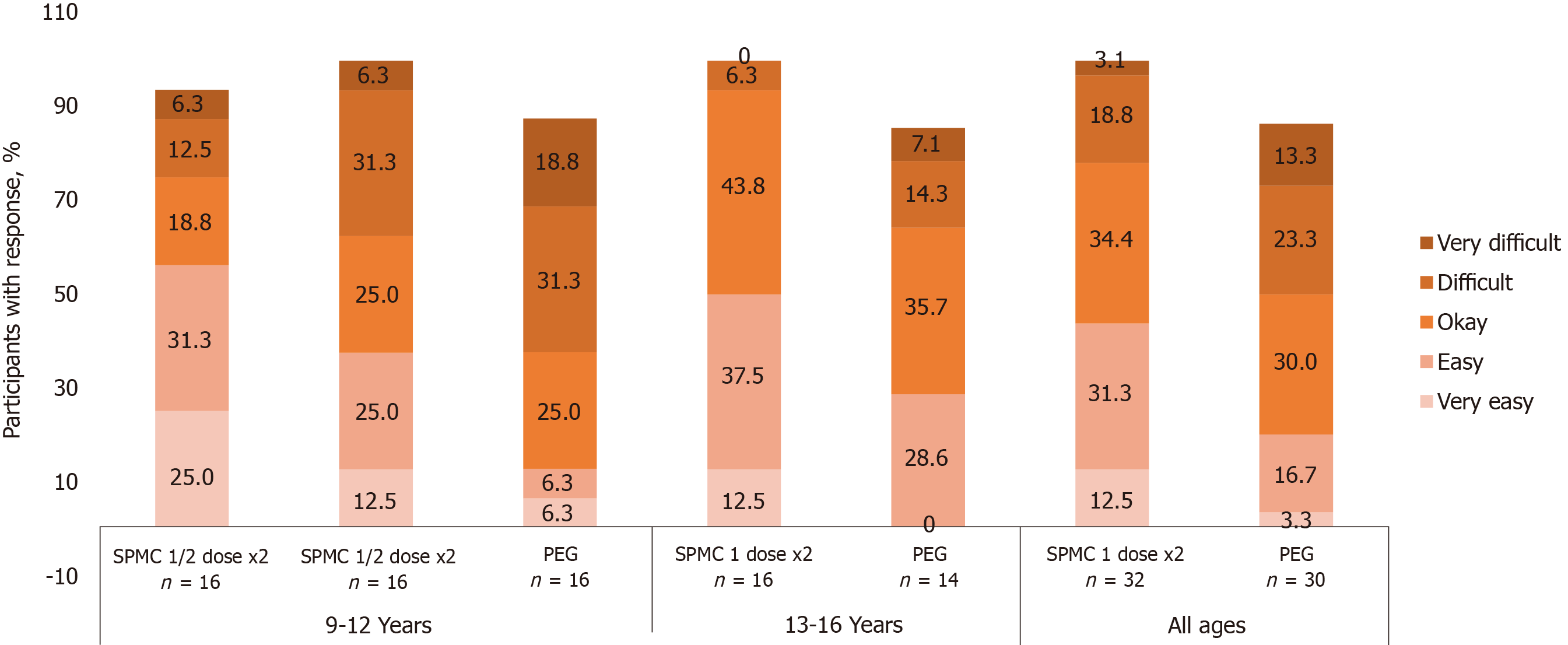Copyright
©The Author(s) 2020.
World J Gastroenterol. Oct 28, 2020; 26(40): 6260-6269
Published online Oct 28, 2020. doi: 10.3748/wjg.v26.i40.6260
Published online Oct 28, 2020. doi: 10.3748/wjg.v26.i40.6260
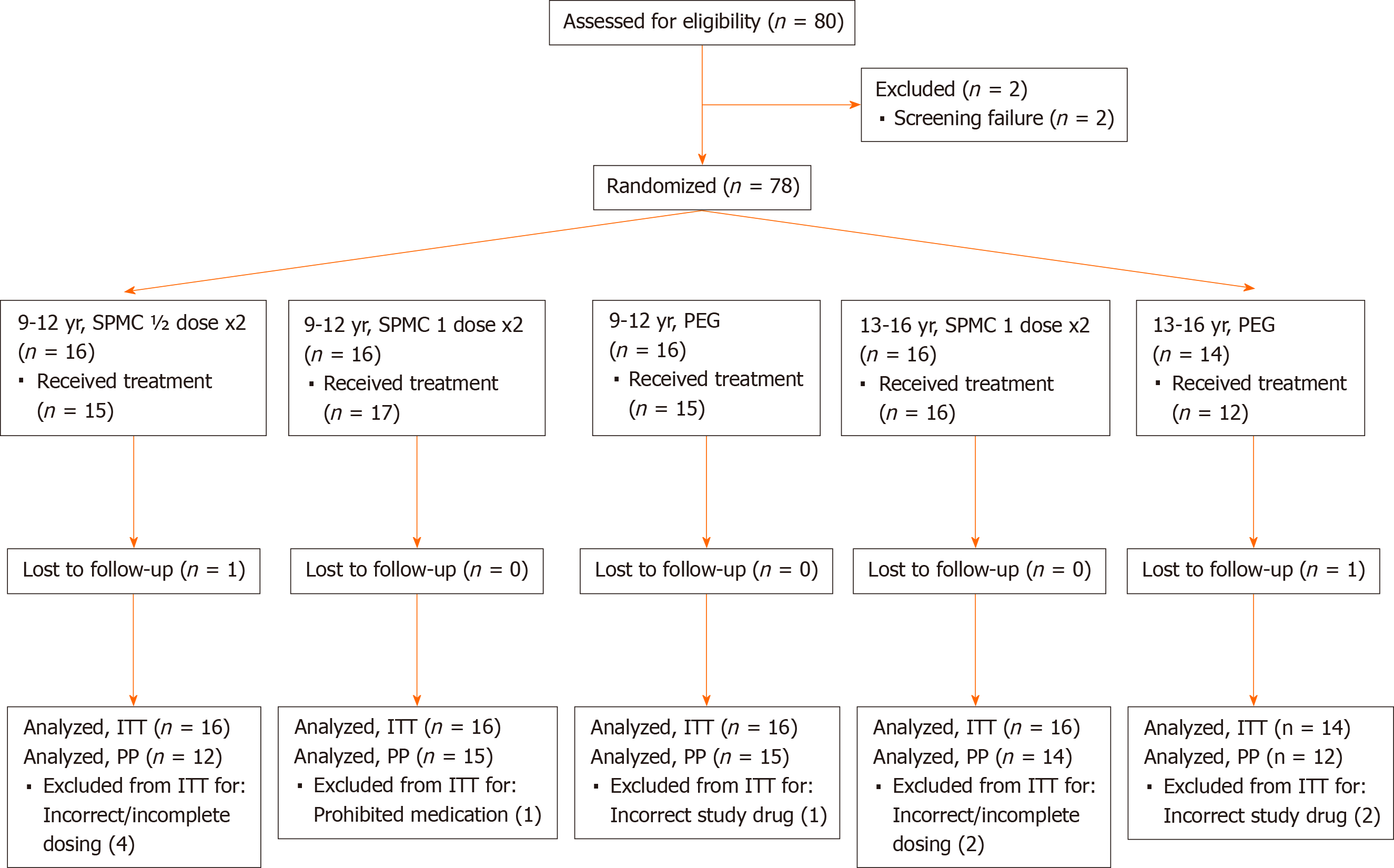
Figure 1 Consort diagram of study population.
One participant in the SPMC ½ dose x2 group received SPMC 1 dose x2 treatment. ITT: Intent-to-treat; PEG: Polyethylene glycol; PP: Per protocol; SPMC; Sodium picosulfate, magnesium oxide, and citric acid.
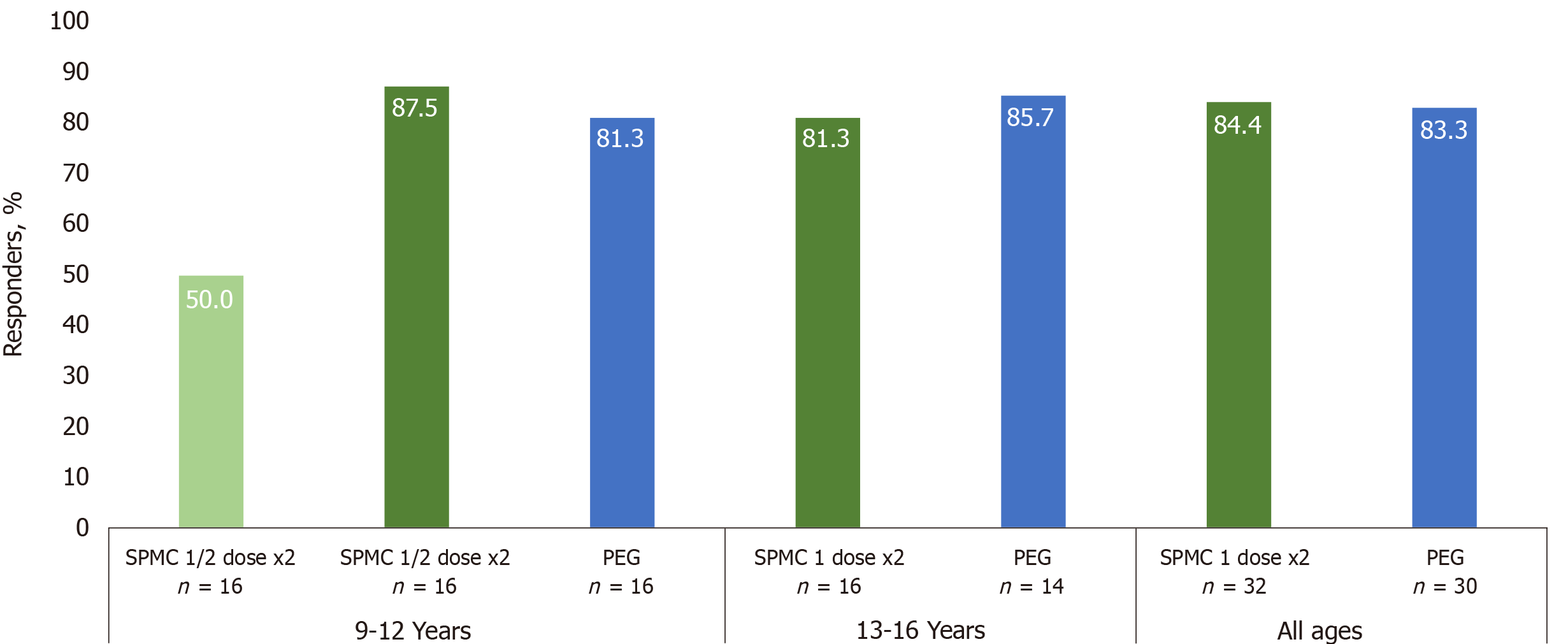
Figure 2 The majority of participants receiving sodium picosulfate, magnesium oxide, and citric acid 1 dose x2 in both age groups were responders for overall colon cleansing on the modified Aronchick scale (AS; ‘excellent’ or ‘good’), rated by a treatment-blinded endoscopist.
The responder rates of SPMC 1 dose x2 group were similar to PEG group. SPMC; Sodium picosulfate, magnesium oxide, and citric acid; PEG: Polyethylene glycol.
Figure 3 Participants were asked “How easy was it to drink the bowel cleanout regimen?”.
Overall, 43.8% of participants receiving SPMC 1 dose x2 reported it was ‘very easy’ or ‘easy’ to drink, compared with 20.0% receiving PEG.
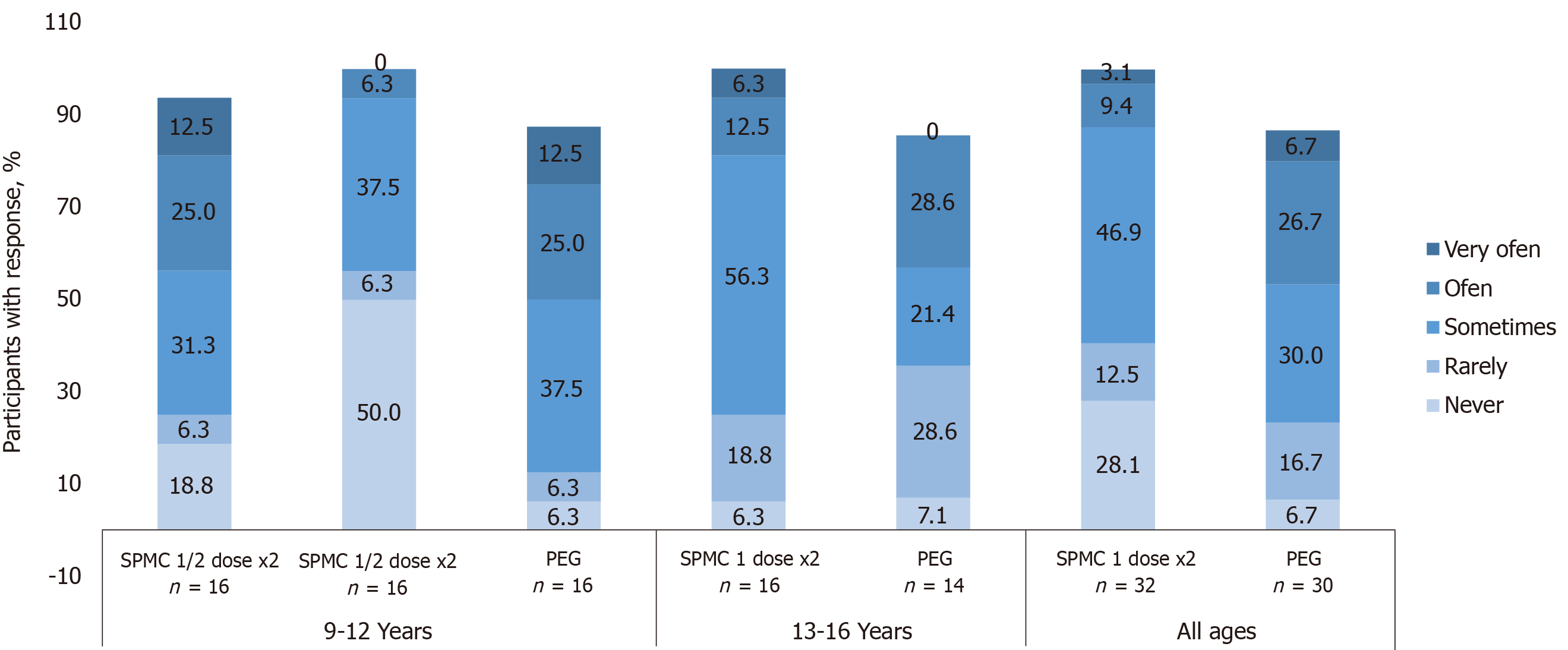
Figure 4 Participants were asked “How often did your tummy hurt since you started the cleanout?”.
28% of participants receiving sodium picosulfate, magnesium oxide, and citric acid (SPMC) 1 dose x2 reported ‘never’ hurting, compared with 6.7% receiving polyethylene glycol (PEG). Only 12.5% of those receiving SPMC 1 dose x2 reported abdominal discomfort ‘often’ or ‘very often’, whereas 33.4% receiving PEG did. Participants with no response are not shown on the graphs and, therefore, numbers may not add to 100%. SPMC: Sodium picosulfate, magnesium oxide, and citric acid; PEG: Polyethylene glycol.
- Citation: Cuffari C, Ciciora SL, Ando M, Boules M, Croffie JM. Pediatric bowel preparation: Sodium picosulfate, magnesium oxide, citric acid vs polyethylene glycol, a randomized trial. World J Gastroenterol 2020; 26(40): 6260-6269
- URL: https://www.wjgnet.com/1007-9327/full/v26/i40/6260.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i40.6260