Copyright
©The Author(s) 2020.
World J Gastroenterol. Oct 14, 2020; 26(38): 5896-5910
Published online Oct 14, 2020. doi: 10.3748/wjg.v26.i38.5896
Published online Oct 14, 2020. doi: 10.3748/wjg.v26.i38.5896
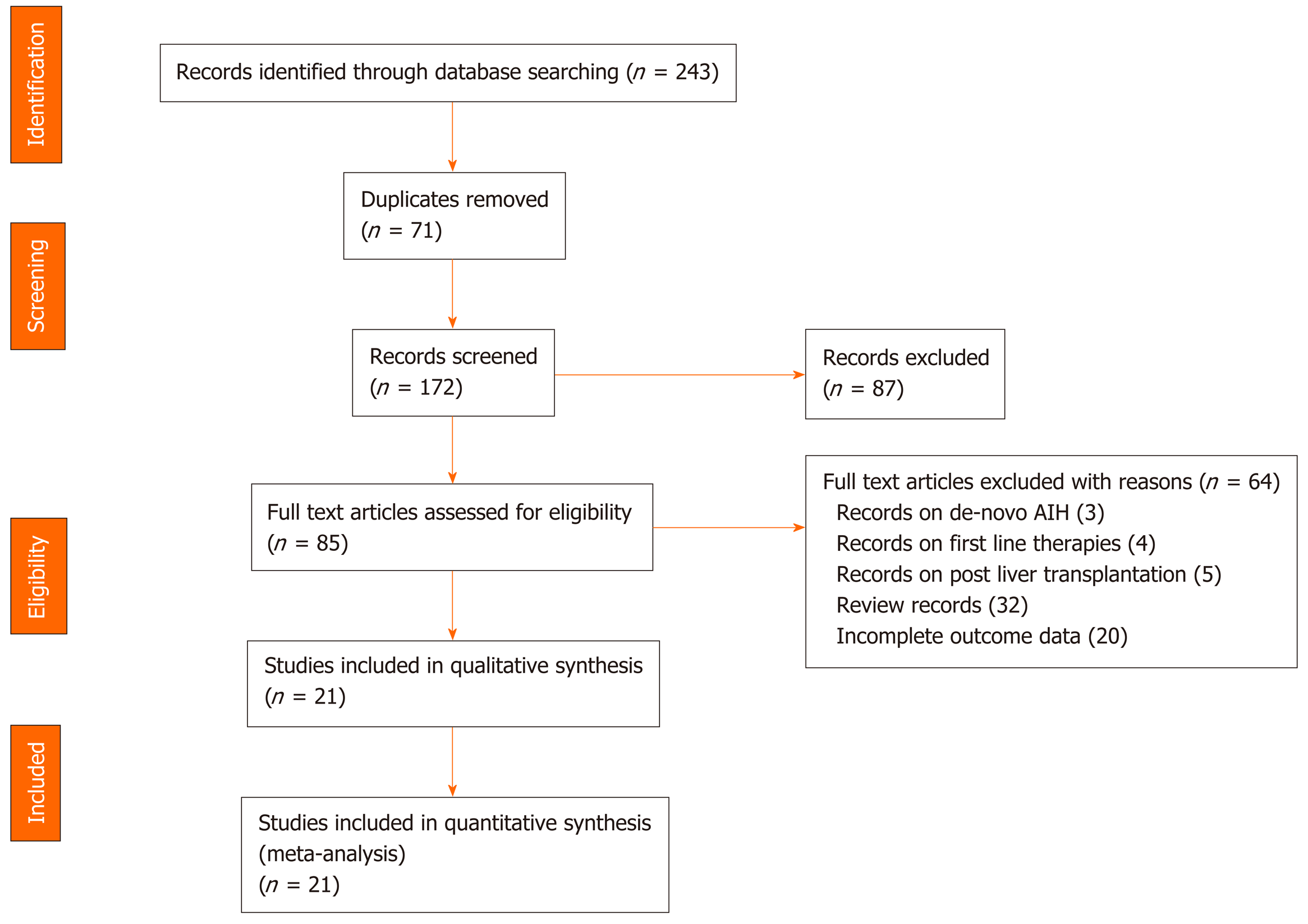
Figure 1 PRISMA flow diagram of the articles retrieved by systematic literature search.
AIH: Autoimmune hepatitis.
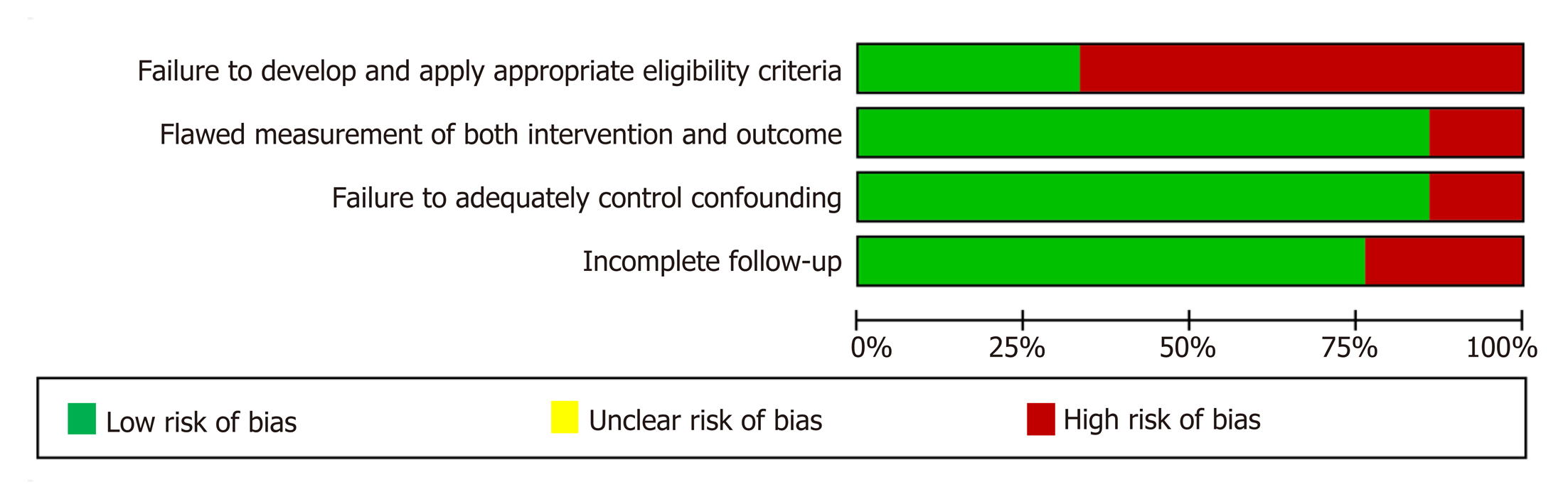
Figure 2 Assessment of methodological quality (risk of bias) of articles identified in the systematic literature search and included in our meta-analysis.
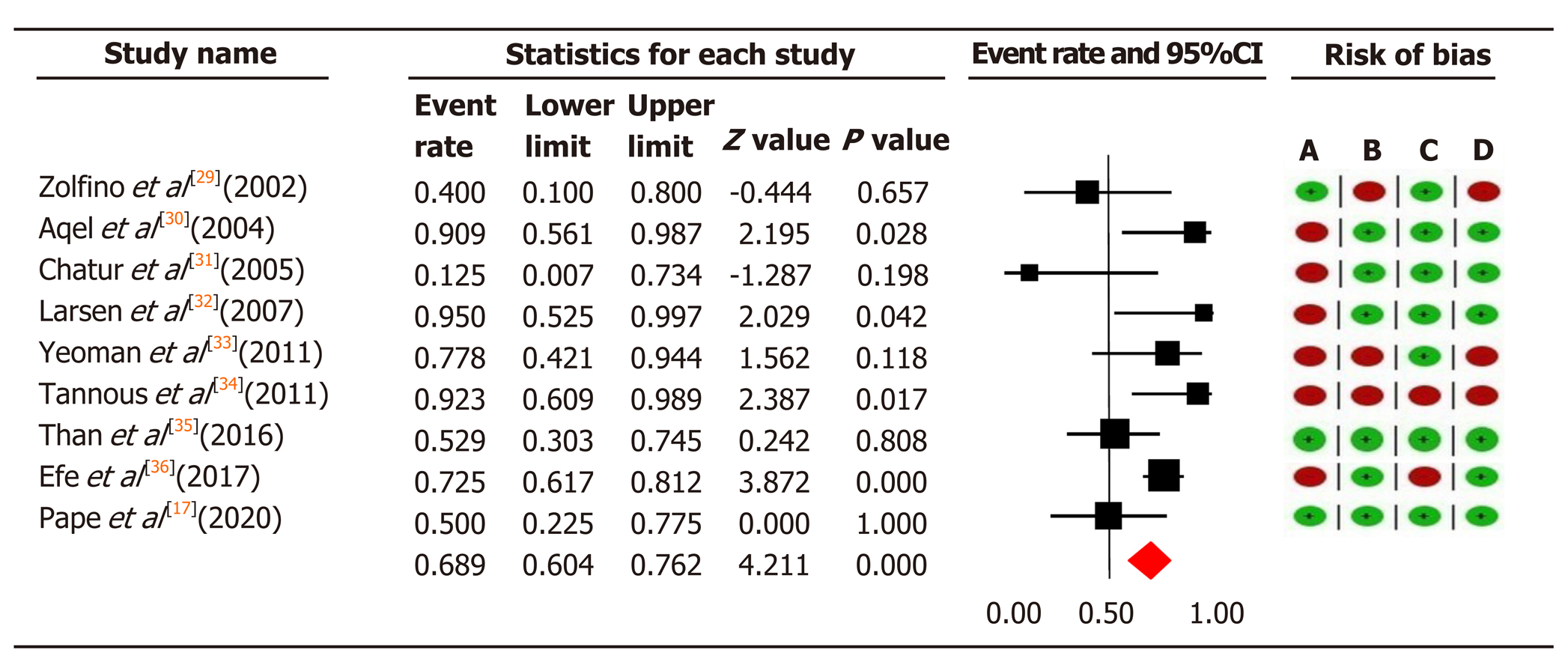
Figure 3 The pooled event rate of biochemical remission in the tacrolimus group with risk of bias assessment per study.
Heterogeneity: Q = 16.25, degree of freedom = 8 (P = 0.039); I2 = 50.76%. Test for overall effect: Z = 4.21 (P < 0.0001). A: Failure to develop and apply appropriate eligibility criteria (inclusion of control population); B: Flawed measurement of both exposure and outcome; C: Failure to adequately control confounding; D: Incomplete follow-up. CI: Confidence interval.
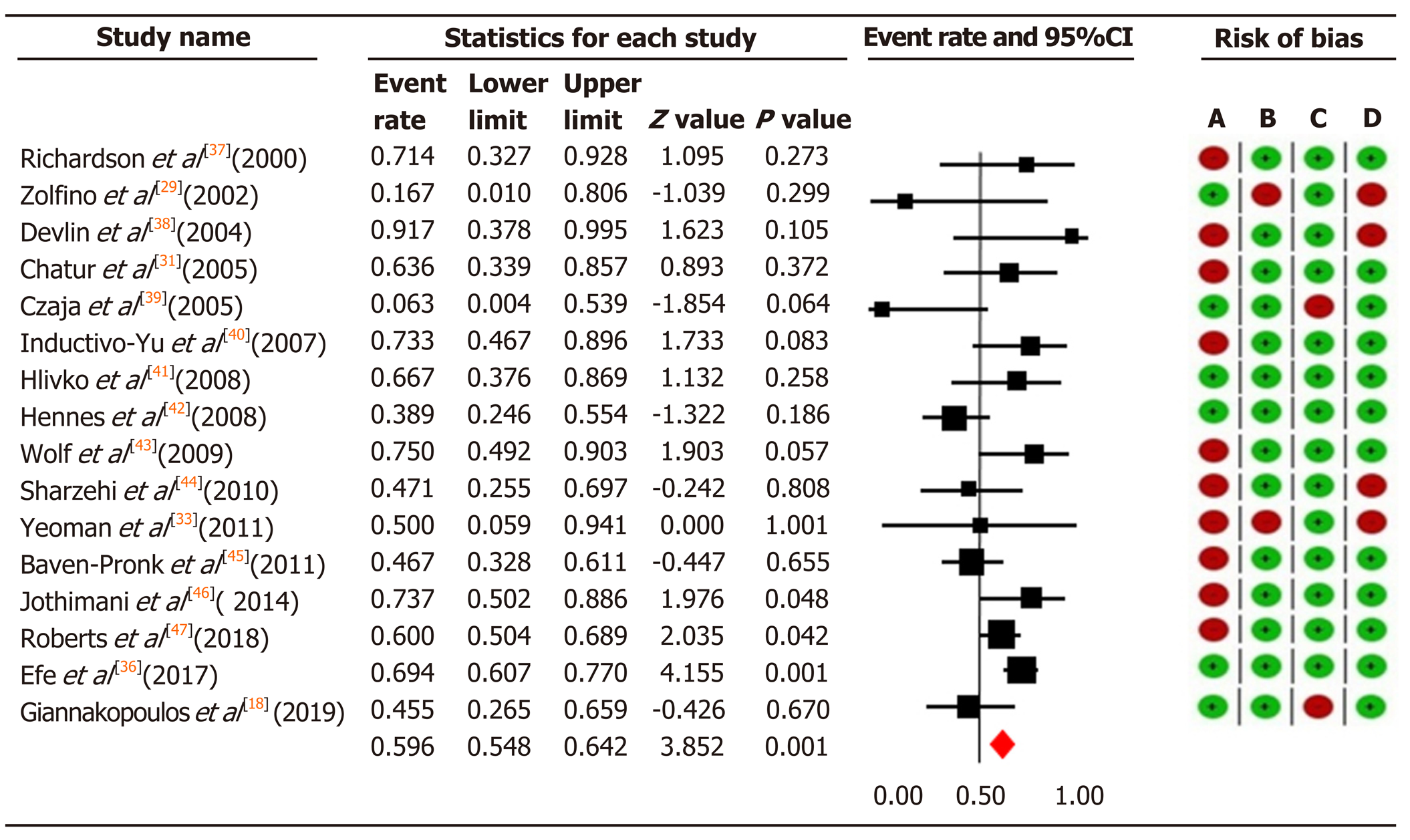
Figure 4 The pooled event rate of biochemical remission in the mycophenolate mofetil group with risk of bias assessment per study.
Heterogeneity: Q = 29.72, degree of freedom = 15 (P = 0.013); I2 = 49.52%. Test for overall effect: Z = 3.85 (P = 0 < 0.0001). A: Failure to develop and apply appropriate eligibility criteria (inclusion of control population); B: Flawed measurement of both exposure and outcome; C: Failure to adequately control confounding; D: Incomplete follow-up. CI: Confidence interval.
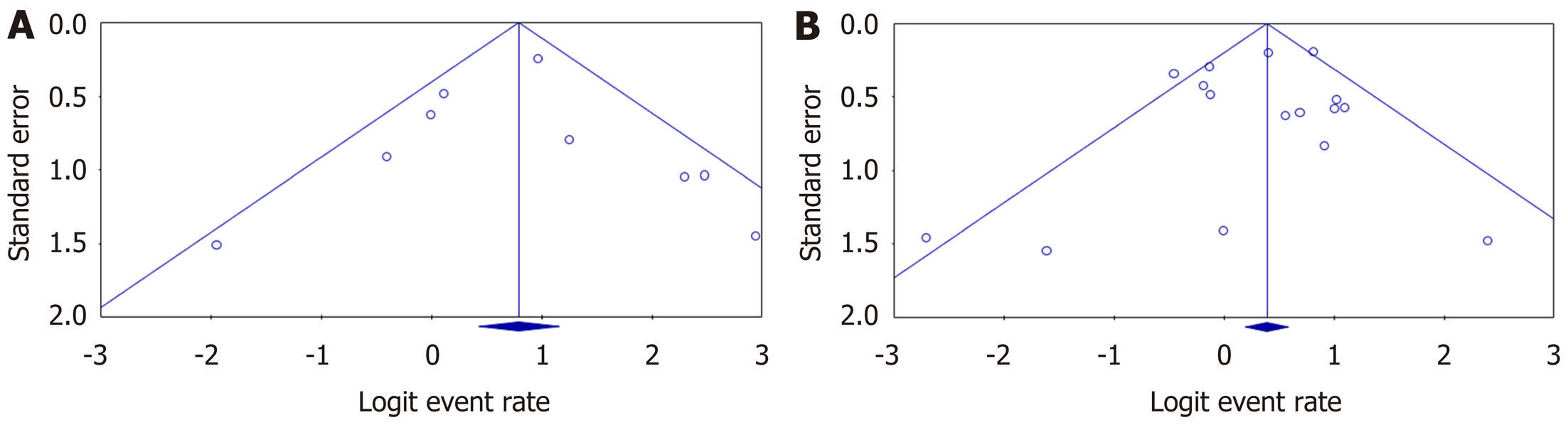
Figure 5 The publication bias of included studies for biochemical remission in (A) tacrolimus and (B) mycophenolate mofetil groups.
- Citation: Abdollahi M, Khalilian Ekrami N, Ghojazadeh M, Boezen HM, Somi M, Alizadeh BZ. Tacrolimus and mycophenolate mofetil as second-line treatment in autoimmune hepatitis: Is the evidence of sufficient quality to develop recommendations? World J Gastroenterol 2020; 26(38): 5896-5910
- URL: https://www.wjgnet.com/1007-9327/full/v26/i38/5896.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i38.5896