Copyright
©The Author(s) 2020.
World J Gastroenterol. Sep 7, 2020; 26(33): 4900-4918
Published online Sep 7, 2020. doi: 10.3748/wjg.v26.i33.4900
Published online Sep 7, 2020. doi: 10.3748/wjg.v26.i33.4900
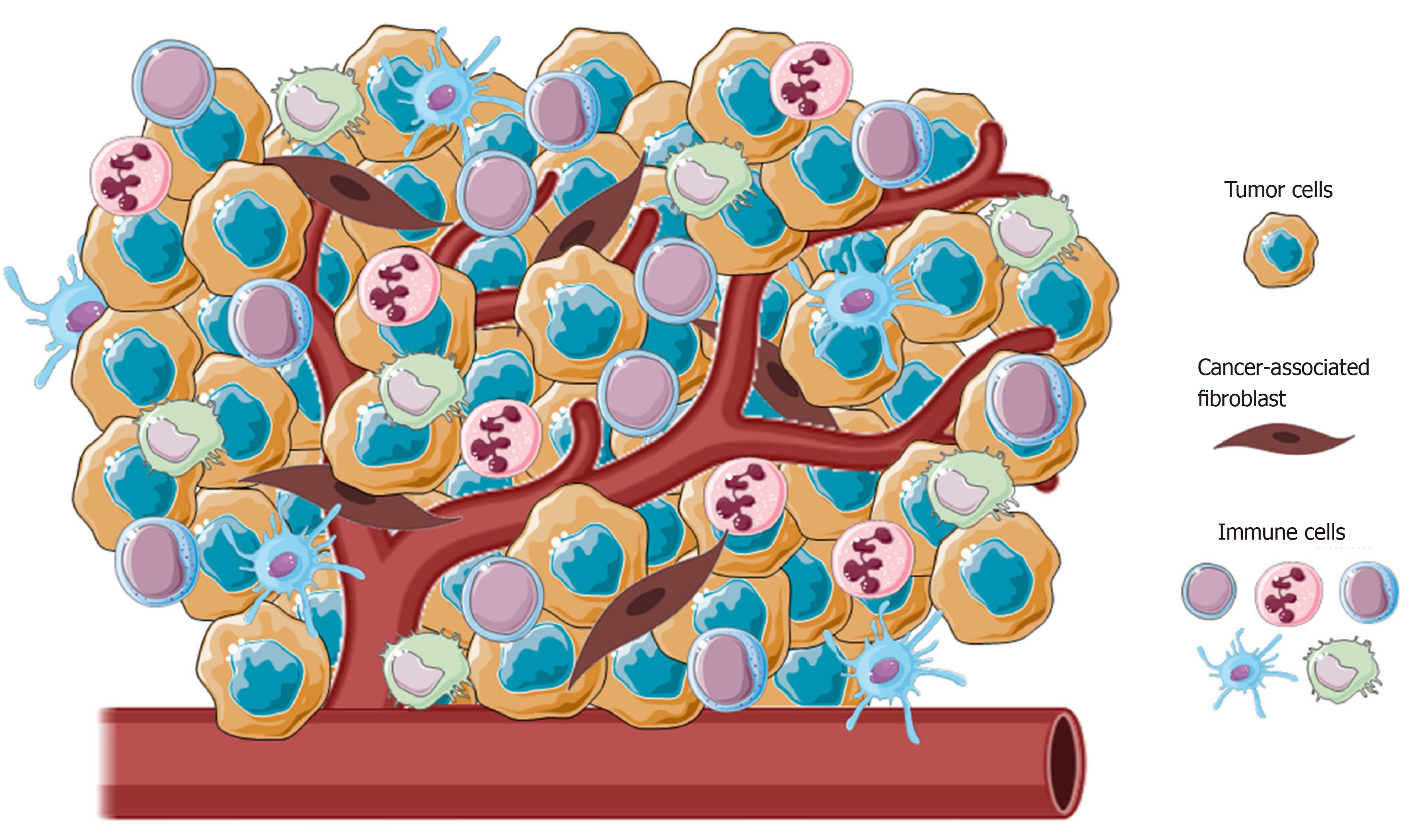
Figure 1 Representative image of the main cellular component of tumor microenvironment.
Tumors present a wide array of cells from both innate and adaptative immunity (i.e., macrophages, natural killer cells, neutrophils and lymphocytes) able to foster cancer growth and malignancy.
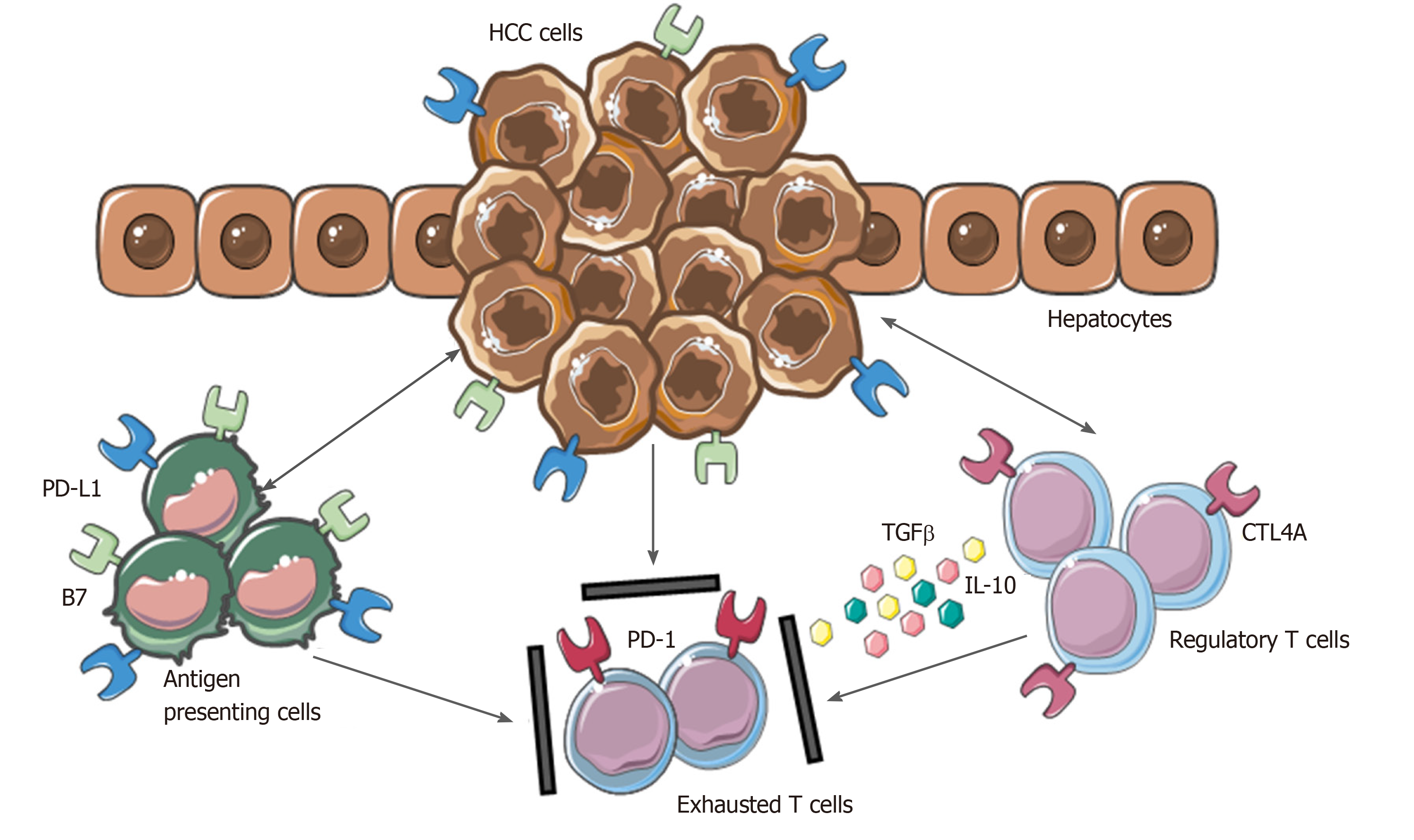
Figure 2 Mechanisms involved in hepatocellular carcinoma immune evasion.
In physiological conditions, liver has the ability to induce immunotolerance against antigen from gastrointestinal tract. These mechanisms have a detrimental role during hepatocellular carcinoma development and progression. Upregulation of inhibitory programmed death-ligand 1 molecule from tumor cells, Kupffer cells, liver sinusoidal endothelial cells and antigen presenting cells, together with the release of interleukin-10 and transforming growth factor beta, lead to an exhausted phenotype of CD8+ cells and prevent tumor cells from immune damage. HCC: Hepatocellular carcinoma; PD-L1: Programmed death-ligand 1; CTL4A: Cytotoxic T lymphocyte antigen 4; PD-1: Programmed cell death protein 1; TGFβ: Transforming growth factor beta; IL-10: Interleukin-10.
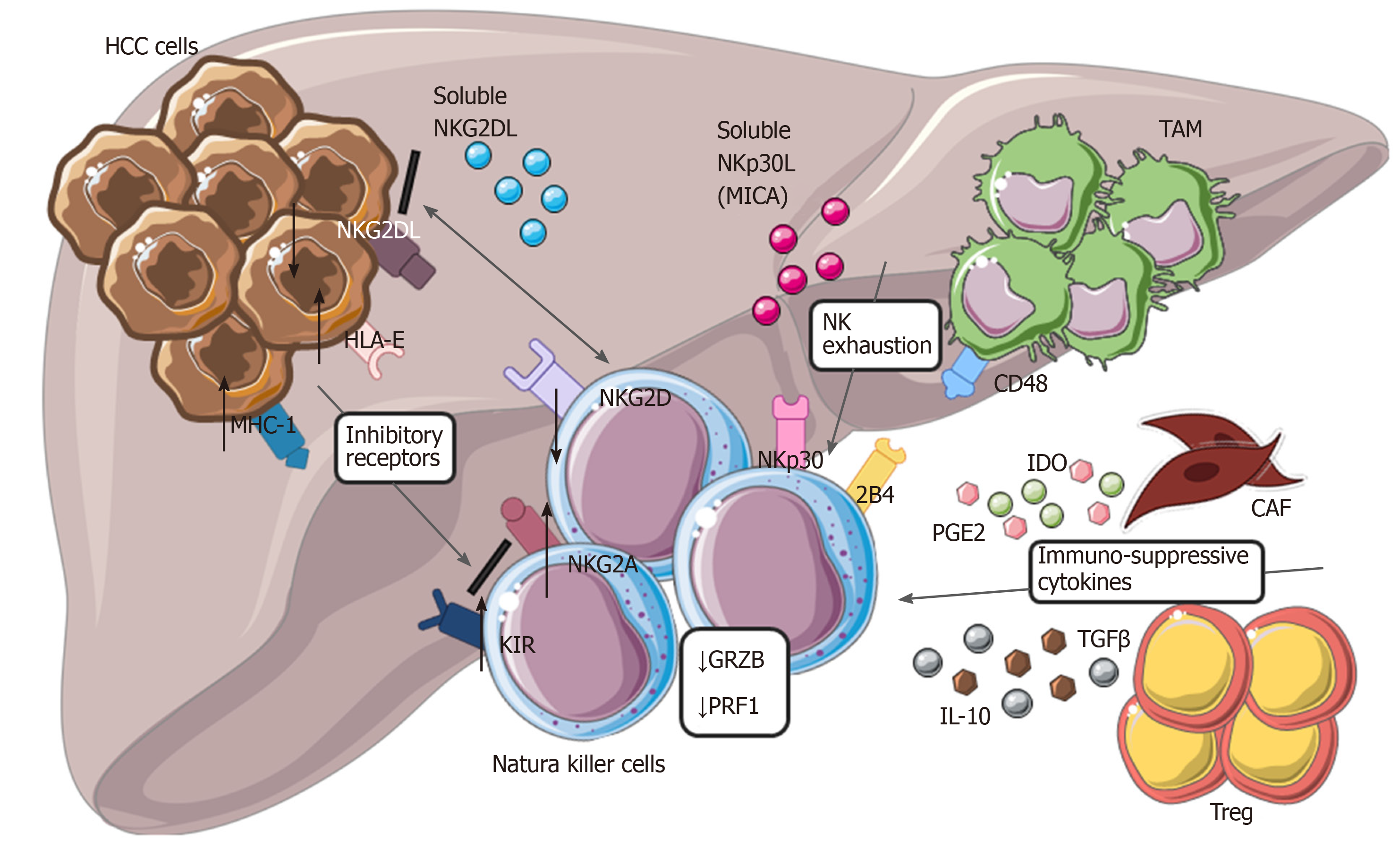
Figure 3 Modulation of natural killer cells cytotoxic activity in hepatocellular carcinoma.
Tumor cells with tumor-associated macrophages and other cells within tumor microenvironment are involved in dysfunctional activity of natural killer cells (NK), reducing their ability to recognize and eliminate malignant cells. Down regulation of NKG2D, up-regulation of different inhibitory receptors, secretion of cytokines from cancer-associated fibroblasts and Treg, interaction of CD48/2B4 are the main mechanisms involved in NK exhaustion. HCC: Hepatocellular carcinoma; NK: Natural killer cells; CAF: Cancer-associated fibroblasts; TAM: Tumor-associated macrophages; IL-10: Interleukin-10; MHC-1: major histocompatibility complex class I; KIR: Killer Ig-like receptors.
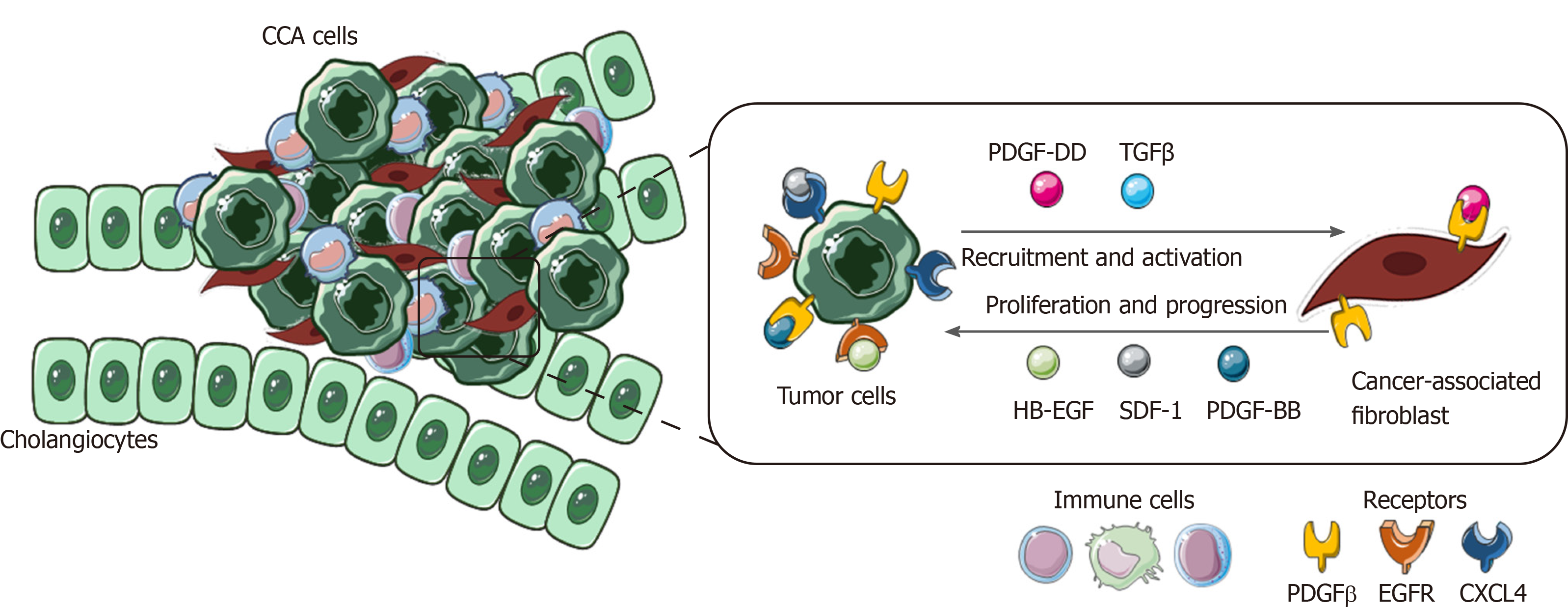
Figure 4 Role of cancer-associated fibroblasts in cholangiocarcinoma.
In the figure is shown the paracrine loop between tumor cells and cancer-associated fibroblasts, the main cellular component of cholangiocarcinoma tumor microenvironment (TME). Several survival and recruitment signals are exchanged within TME, leading to tumor growth and progression. CCA: Cholangiocarcinoma; TGFβ: Transforming growth factor beta; PDGF-β: Platelet-derived growth factor receptor beta; EGFR: Endothelial growth factor receptor.
- Citation: Polidoro MA, Mikulak J, Cazzetta V, Lleo A, Mavilio D, Torzilli G, Donadon M. Tumor microenvironment in primary liver tumors: A challenging role of natural killer cells. World J Gastroenterol 2020; 26(33): 4900-4918
- URL: https://www.wjgnet.com/1007-9327/full/v26/i33/4900.htm
- DOI: https://dx.doi.org/10.3748/wjg.v26.i33.4900