Copyright
©The Author(s) 2019.
World J Gastroenterol. Feb 7, 2019; 25(5): 521-538
Published online Feb 7, 2019. doi: 10.3748/wjg.v25.i5.521
Published online Feb 7, 2019. doi: 10.3748/wjg.v25.i5.521
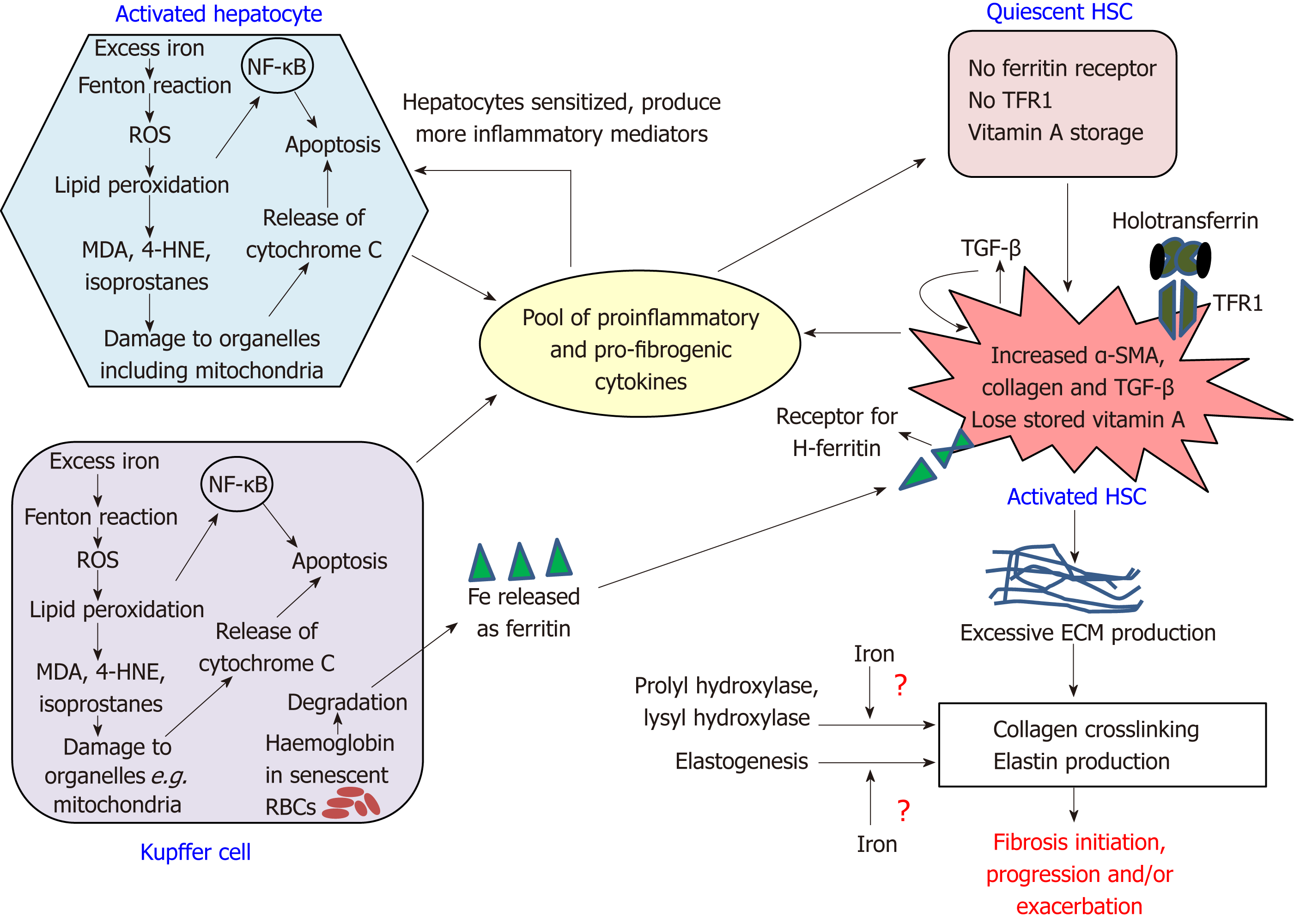
Figure 1 Intercellular network of events in fibrosis.
The figure shows the interactions between hepatocytes, Kupffer cells and hepatic stellate cells that initiate and drive fibrosis progression. The pool of pro-fibrogenic and pro-inflammatory mediators include C-C motif chemokine ligand 5, macrophage inflammatory proteins 1 and 2, monocyte chemoattractant protein-1, tumor necrosis factor alpha, transforming growth factors alpha and beta, platelet-derived growth factor, interleukin (IL)-1β, IL-6, inducible nitric oxide synthase, and protein adducts of malondialdehyde and 4-hydroxynonenal. HNE: Hydroxynonenal; HSC: Hepatic stellate cell; MDA: Malondialdehyde; NF-κB: Nuclear factor kappa B; RBCs: Red blood cells; ROS: Reactive oxygen species; TFR1: Transferrin receptor 1; αSMA: Alpha smooth muscle actin; ECM: Extracellular matrix; TGF-β: Transforming growth factors beta.
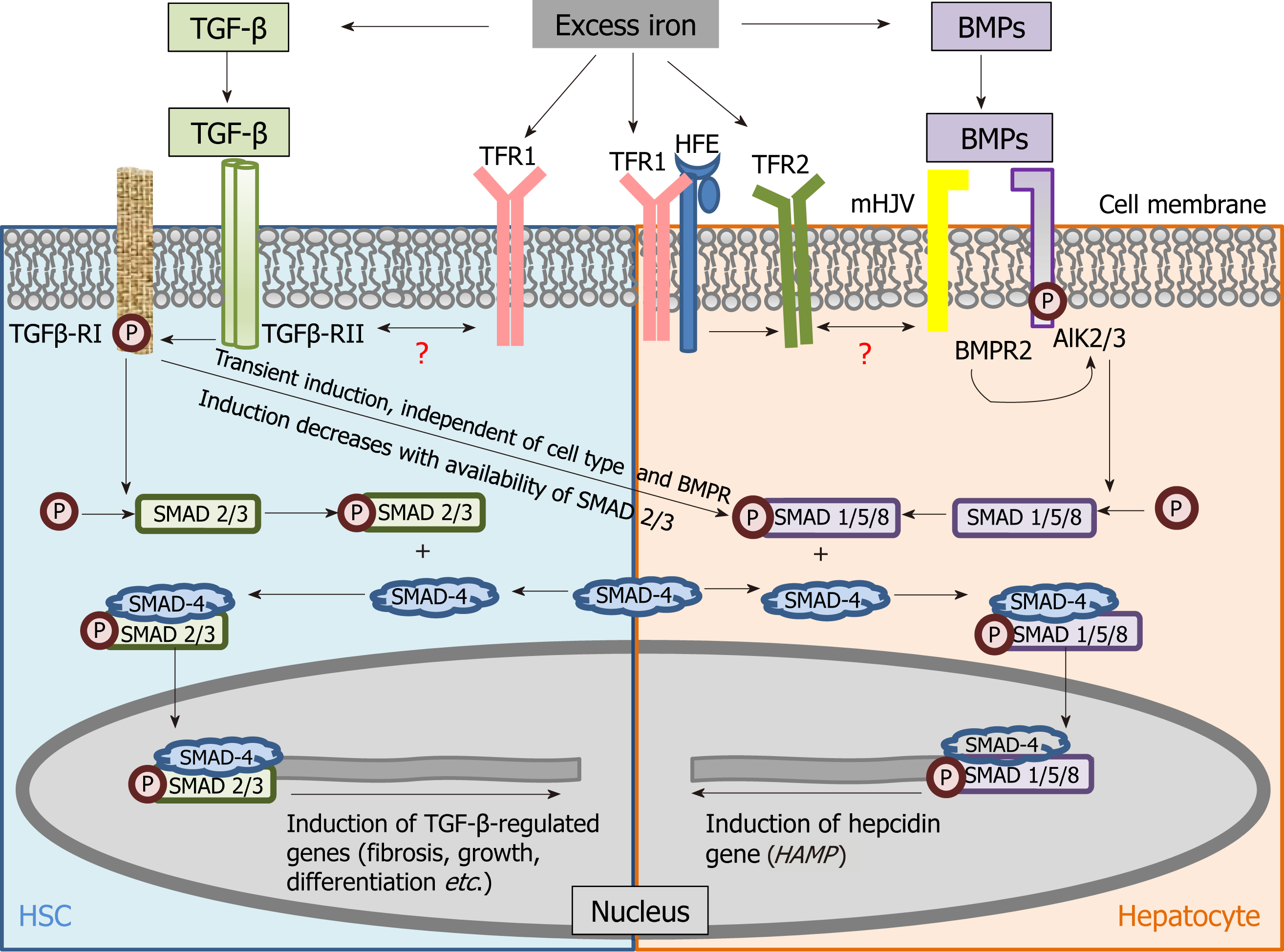
Figure 2 Schematic of mechanistic cross-connection between the transforming growth factor beta pathway and bone morphogenetic protein signaling.
Shared signalling components between transforming growth factor beta (TGF-β) (fibrosis-related) and bone morphogenetic protein (iron-related) pathways have been shown in hepatic stellate cells and hepatocytes. Previous study demonstrated TGF-β-induced hepcidin expression in human macrophages, while Chen et al[44] showed that this occurred through TGF-β-RII/RI in mouse and human hepatocytes via the non-canonical pathway involving small mothers against decapentaplegic protein-1/5/8 phosphorylation. ALK: Activin receptor-like kinase; BMPR: Bone morphogenetic protein receptor; HFE: High iron protein; HSC: Hepatic stellate cell; mHJV: Membrane-bound hemojuvelin protein; P: Phosphorylation; SMAD: Small mothers against decapentaplegic protein; TFR: Transferrin receptor; TGF-β-R: Transforming growth factor receptor.
- Citation: Mehta KJ, Farnaud SJ, Sharp PA. Iron and liver fibrosis: Mechanistic and clinical aspects. World J Gastroenterol 2019; 25(5): 521-538
- URL: https://www.wjgnet.com/1007-9327/full/v25/i5/521.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i5.521