Copyright
©The Author(s) 2019.
World J Gastroenterol. Sep 28, 2019; 25(36): 5469-5482
Published online Sep 28, 2019. doi: 10.3748/wjg.v25.i36.5469
Published online Sep 28, 2019. doi: 10.3748/wjg.v25.i36.5469
Figure 1 Photomicrographs of hematoxylin and eosin staining.
There was no difference with regard to morphology between control (A), IBS (B), IBS + C. butyricum (C), and IBS + NS (D) tissues in hematoxylin and eosin staining (original magnification, ×200) (n = 6 per group).
Figure 2 Effects of Clostridium butyricum on visceral hypersensitive in TNBS-induced irritable bowel syndrome mice.
The abdominal withdrawal reflex (AWR) scores at 0.25 mL (A), 0.35 mL (B), and 0.50 mL (C) distension volumes were measured on day 28. Data are expressed as the mean ± SEM (n = 6). aP < 0.05, bP < 0.05, cP < 0.05 vs control group, IBS + normal saline group, and IBS group, respectively. IBS: Irritable bowel syndrome.
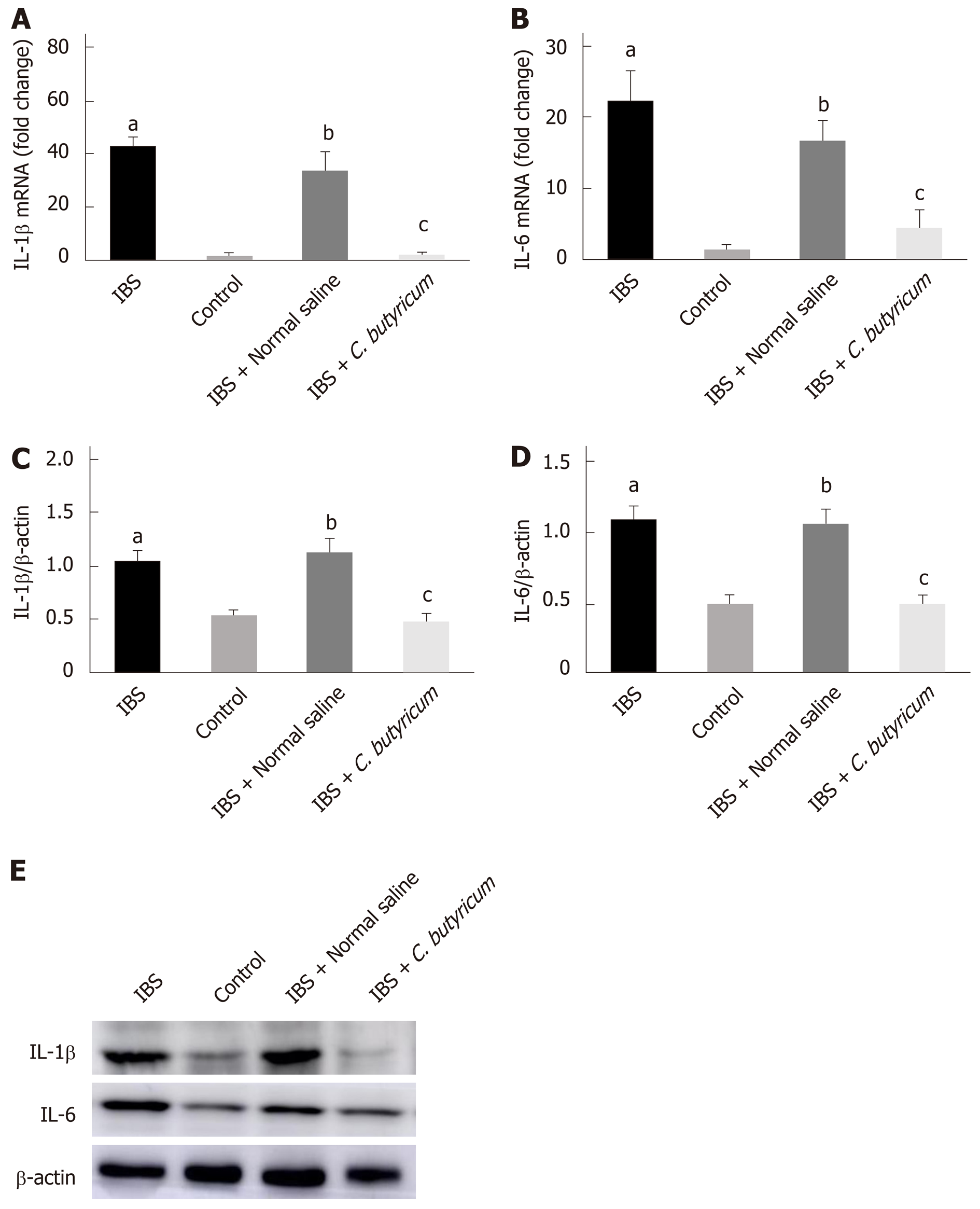
Figure 3 Effects of Clostridium butyricum on the production of proinflammatory cytokines in irritable bowel syndrome mice.
Real-time PCR (A and B) and Western blot analysis(C-E) were used to measure the levels of proinflammatory cytokines IL-1β and IL-6. The results showed that IL-1β and IL-6 in the intestinal low-grade inflammatory state of IBS were significantly higher than those in the normal control group. The secretion of proinflammatory cytokines was significantly decreased after C. butyricum intervention. aP < 0.05, bP < 0.05, cP < 0.05 vs control group, IBS + normal saline group, and IBS group, respectively. IBS: Irritable bowel syndrome.
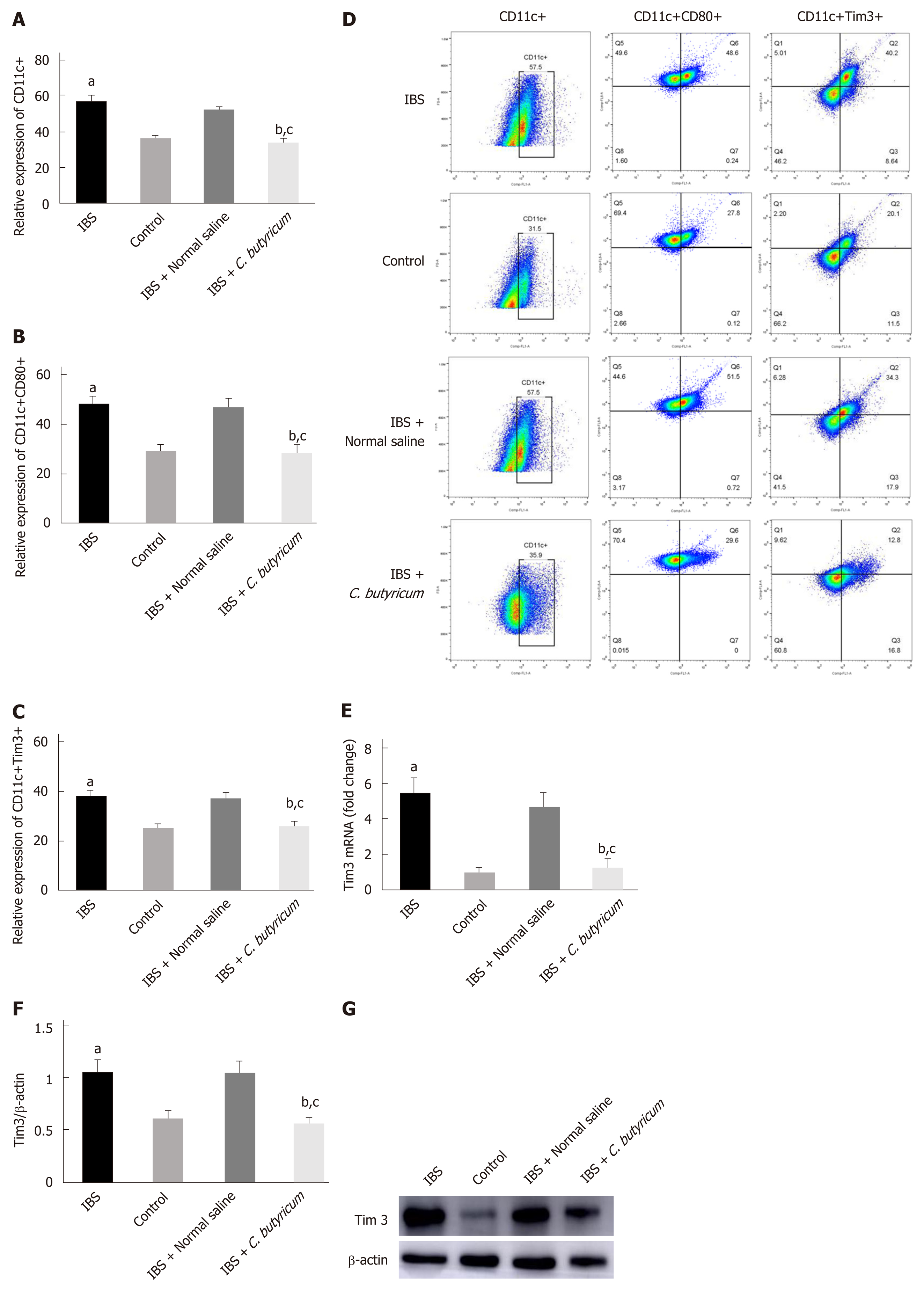
Figure 4 Effects of Clostridium butyricum on the quantity and functional status of the lamina propria dendritic cells.
A: Flow cytometry of CD11c+ LPDCs expression; B: Flow cytometry of CD11C+CD80+ LPDCs expression; C: Flow cytometry of CD11c+Tim3+ LPDCs expression; D: Flow scatter plot of all test indicators; E: Tim-3 mRNA levels measured by real-time PCR; F: Tim3 protein expression measured by Western blot analysis. The results showed that the expression of CD11c+, CD11C+CD80+, and CD11c+Tim3+ LPDCs in the intestinal inflammatory state of IBS was significantly higher than in that in the normal control group, and the costimulatory molecule CD80 representing DC in the activated mature state was also significantly increased (P < 0.01). However, the above indicators were significantly decreased after C. butyricum intervention. Our experimental results suggest that the LPDCs participate in and promote the intestinal inflammatory immune response of IBS and C. butyricum suppresses intestinal inflammation by reducing the number, function, and immunoregulatory molecule Tim3 of LPDCs in IBS mice. aP < 0.05, bP < 0.05, cP < 0.05 vs control group, IBS + normal saline group, and IBS group, respectively. IBS: Irritable bowel syndrome; LPDCs: Lamina propria dendritic cells.
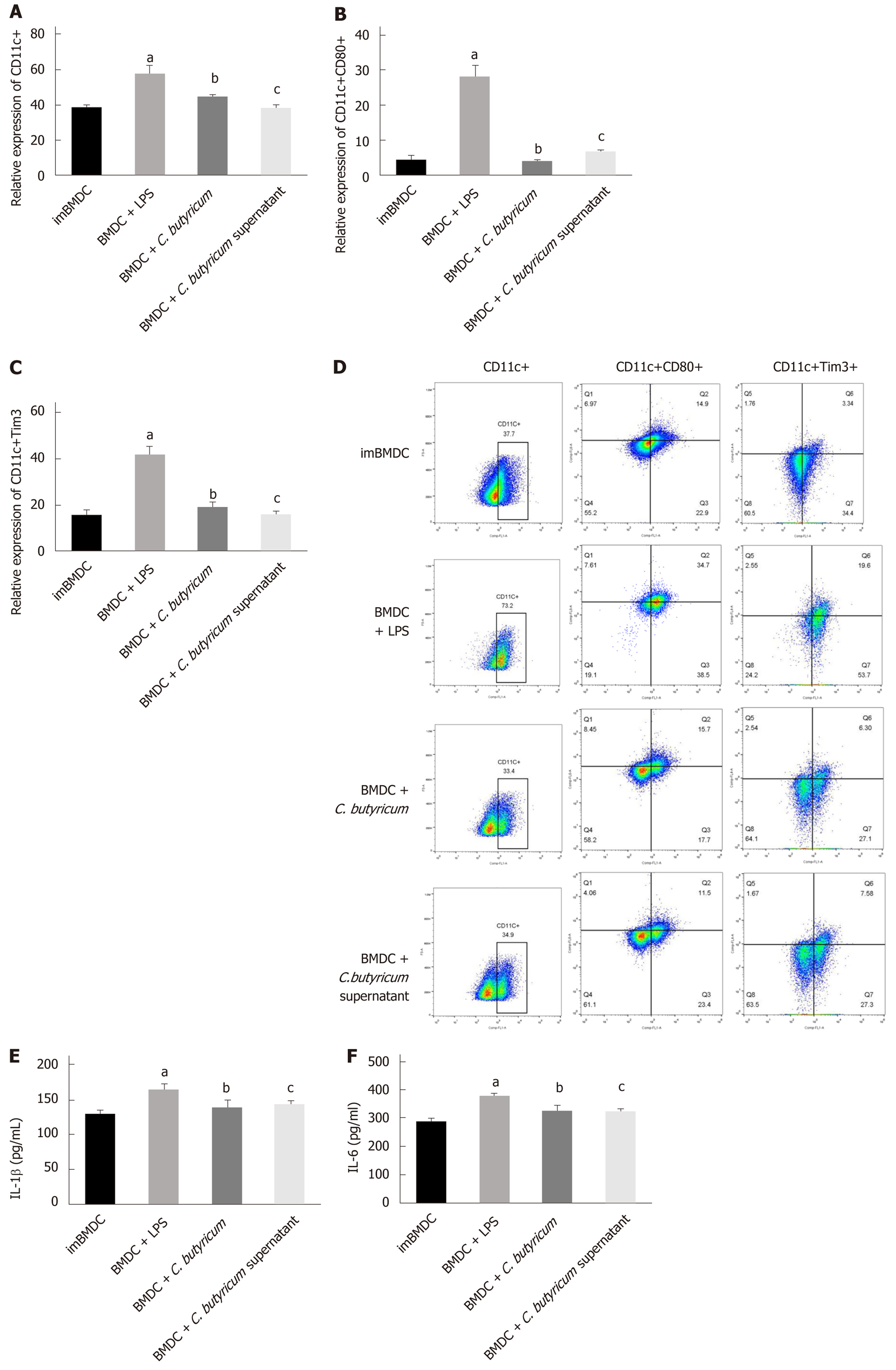
Figure 5 Effects of Clostridium butyricum on the expression of Tim3 and inflammatory cytokines in bone marrow-derived dendritic cells.
LPS stimulated the proliferation and activation of bone marrow-derived dendritic cells (BMDCs). The expression of CD11c+CD80+ BMDCs and CD11c+Tim3+ BMDCs was significantly higher than that of the immature BMDCs group by flow cytometry (A-D). The inflammatory cytokines IL-1 and IL-6 detected by ELISA were also significantly elevated (E-F). Tim3 was highly expressed in mature activated BMDCs. Different forms of probiotics were co-cultured and stimulated with LPS. The expression of Tim3 was down-regulated with the decrease of inflammatory factors. It can be seen that Tim3 is the key point to regulate the activation of DCs. Clostridium butyricum can alter the activation status and function of DCs by regulating the expression of Tim3. aP < 0.05, bP < 0.05, cP < 0.05 vs ImBMDC group, BDMC group, and BDMC + LPS group, respectively. BMDCs: Bone marrow-derived dendritic cells.
- Citation: Zhao Q, Yang WR, Wang XH, Li GQ, Xu LQ, Cui X, Liu Y, Zuo XL. Clostridium butyricum alleviates intestinal low-grade inflammation in TNBS-induced irritable bowel syndrome in mice by regulating functional status of lamina propria dendritic cells. World J Gastroenterol 2019; 25(36): 5469-5482
- URL: https://www.wjgnet.com/1007-9327/full/v25/i36/5469.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i36.5469