Copyright
©The Author(s) 2019.
World J Gastroenterol. Jun 7, 2019; 25(21): 2539-2548
Published online Jun 7, 2019. doi: 10.3748/wjg.v25.i21.2539
Published online Jun 7, 2019. doi: 10.3748/wjg.v25.i21.2539
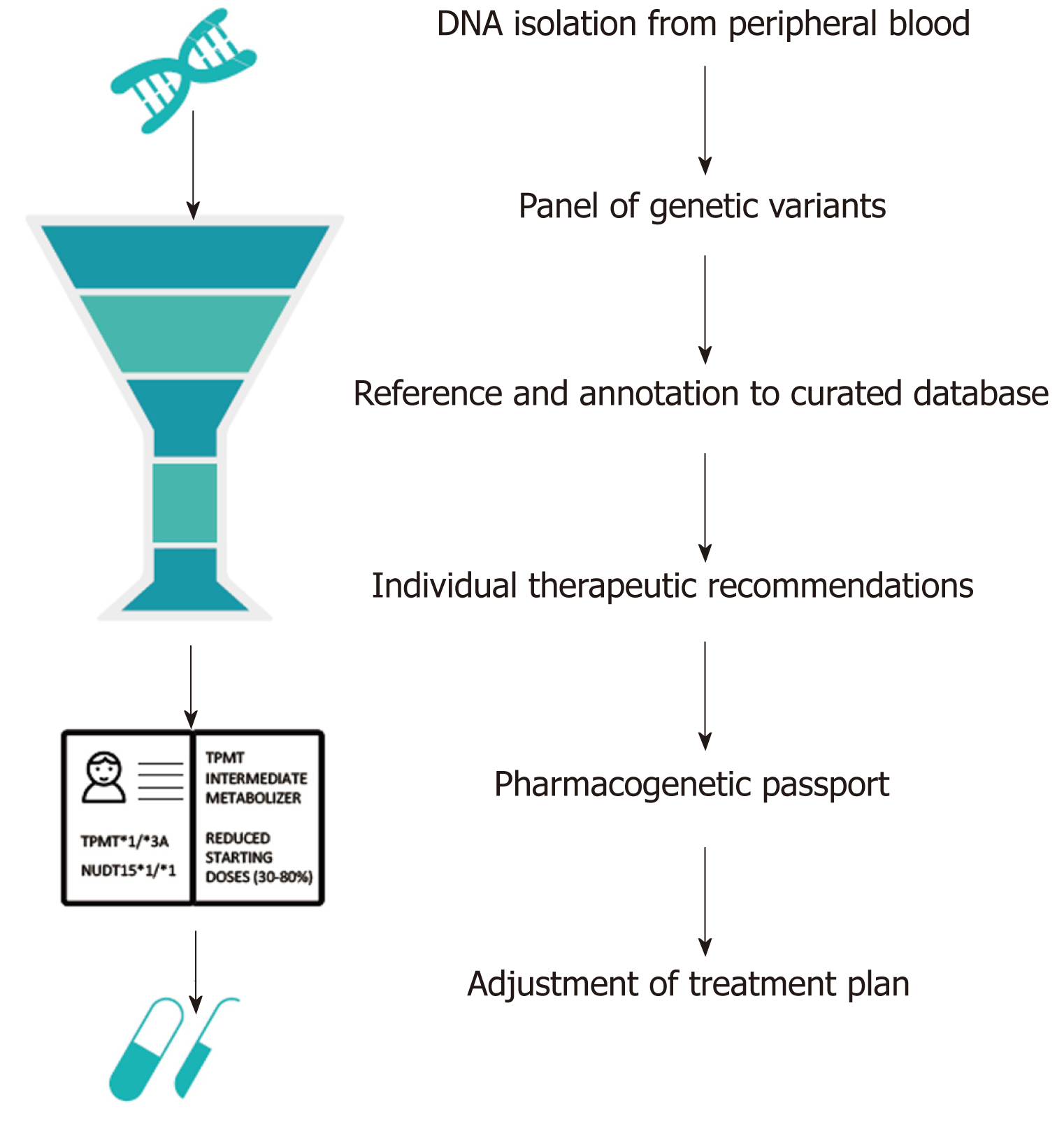
Figure 1 Example of an automated computational pipeline that creates an individual pharmacogenetic passport based on an individual’s genotype.
In this case, the individual is a heterozygous carrier of the loss-of-function TPMT*3A allele and homozygous carrier of the NUDT15*1 allele. Heterozygous carriers of TPMT*3A are at risk for thiopurine-induced myelosuppression due to intermediate TPMT enzyme activity levels. Hence, a reduced dose (30%-80% of target dose) is strongly recommended. Patients with a NUDT15*1/*1 genotype are considered as NUDT15 normal metabolizers[28]. TPMT: Thiopurine S-methyltransferase; NUDT: Nudix hydrolase.
- Citation: Voskuil MD, Bangma A, Weersma RK, Festen EAM. Predicting (side) effects for patients with inflammatory bowel disease: The promise of pharmacogenetics. World J Gastroenterol 2019; 25(21): 2539-2548
- URL: https://www.wjgnet.com/1007-9327/full/v25/i21/2539.htm
- DOI: https://dx.doi.org/10.3748/wjg.v25.i21.2539