Copyright
©The Author(s) 2018.
World J Gastroenterol. Aug 21, 2018; 24(31): 3488-3499
Published online Aug 21, 2018. doi: 10.3748/wjg.v24.i31.3488
Published online Aug 21, 2018. doi: 10.3748/wjg.v24.i31.3488
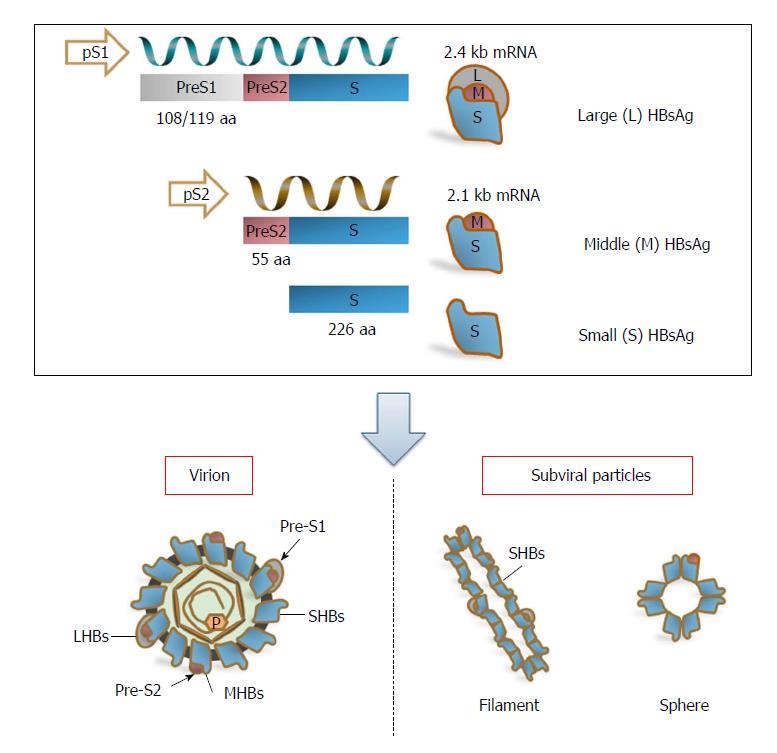
Figure 1 The transcription and expression of hepatitis B virus surface proteins.
The three HBV surface proteins, LHBs, MHBs, and SHBs, are translated from two different mRNAs: LHBs are encoded by the preS1 promoter-initiated 2.4 kb subgenomic RNA; MHBs and SHBs are encoded by the preS2 promoter-initiated 2.1 kb subgenomic RNA. The 2.4 and 2.1 kb subgenomic RNAs share the same 3’ end and only differ in length due to differences at the 5’ end, which lead to different amino-terminal but identical carboxy-terminal regions of the three surface antigens. Therefore, LHBs contain preS1 + preS2 + S (389 or 400 aa residues), MHBs contain preS2 + S (281 aa residues), and SHBs contain the S domain (226 aa residues) alone. For mature/infectious virions, LHBs, MHBs, and SHBs are present in the envelopes at a ratio of approximately 1:1:4. In addition, the major fraction of SHBs forms subviral particles (filaments and spheres) together with the minor parts of LHBs and/or MHBs. HBV: Hepatitis B virus; LHBs: Large surface antigens; MHBs: Middle surface antigens; SHBs: Small surface antigens.
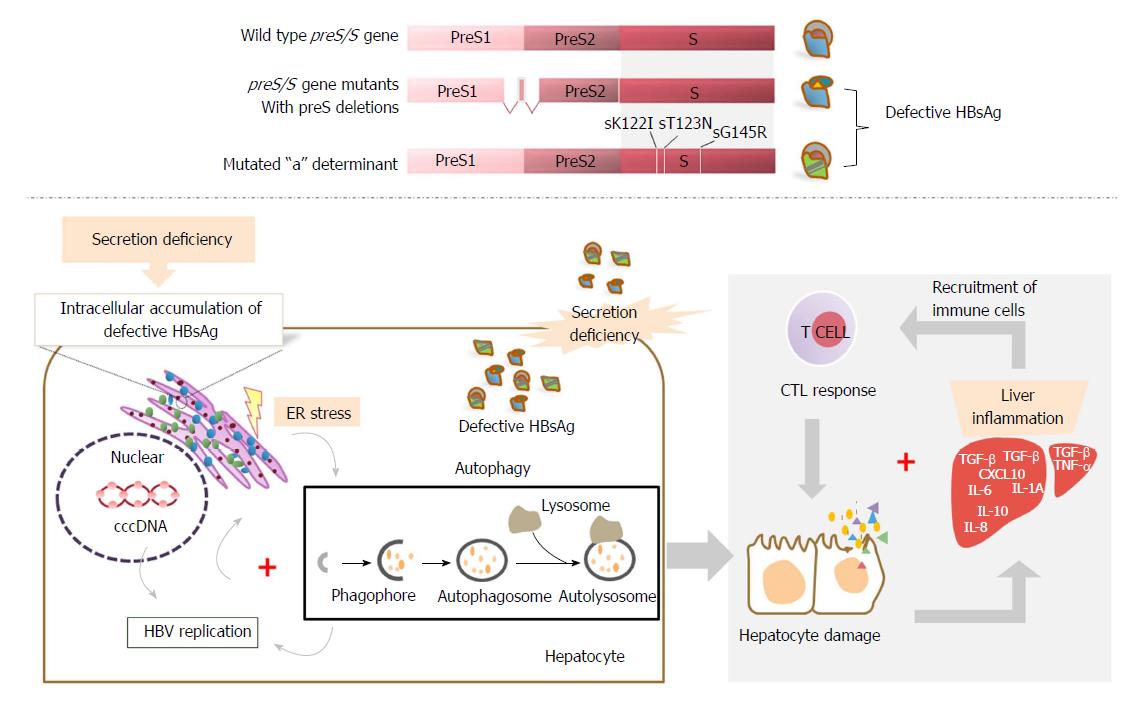
Figure 2 The proposed pathogenic role of mutated secretion-defective hepatitis B surface antigen in fulminant hepatitis.
Defective surface antigens, such as preS deletions and mutations within the “a” determinant, may lead to secretion deficiency of HBsAg. Defective HBsAg can promote covalently closed circular DNA (cccDNA) synthesis and amplification, thus facilitating HBV replication. The intracellular accumulation of defective HBsAg can cause endoplasmic reticulum (ER) stress, subsequently trigger autophagy, and may further enhance HBV replication. The enhanced HBV replication, in turn, leads to accumulation of more defective HBsAg, possibly resulting in a positive feedback with unfavorable outcomes and hepatocyte damages. Inflammation may occur in the liver by recruiting immune cells. Cytotoxic T lymphocyte (CTL) response may be abnormally activated and damage infected hepatocytes, contributing to the progression of fulminant hepatitis. HBsAg: Hepatitis B surface antigen.
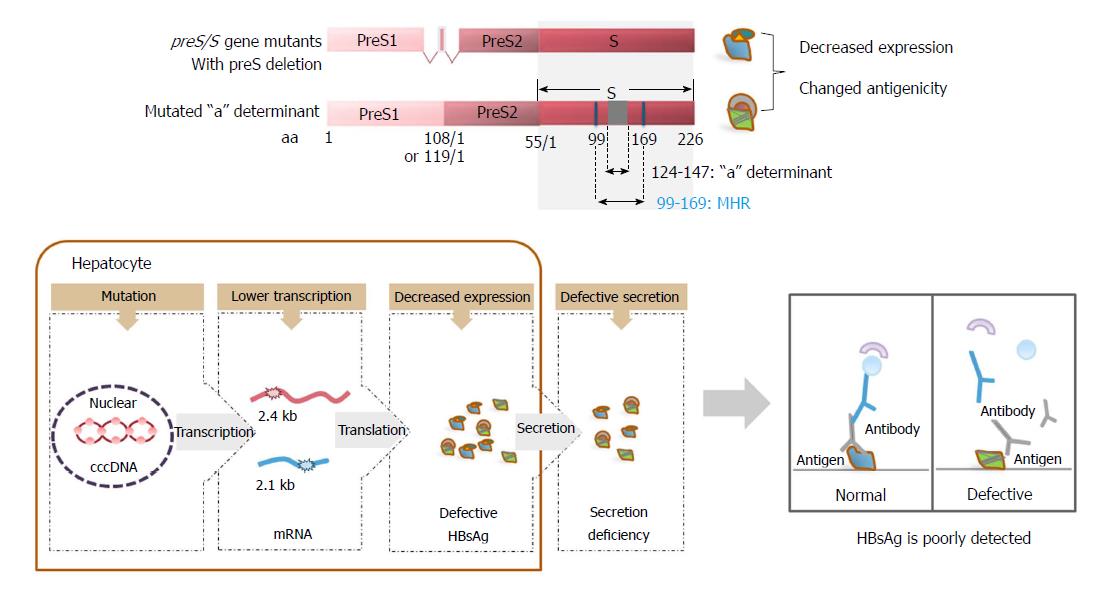
Figure 3 The relationship between the expression of defective surface antigens and occult hepatitis B virus infection.
Surface antigen mutations, such as preS deletions, can impair the transcription of 2.4 and 2.1 kb HBV RNAs, leading to decreased levels of three HBV surface proteins. In addition, defective surface antigens with preS deletions and mutations within the “a” determinant are secretion deficient. Single or multiple mutations occurring within the MHR between the aa residues 99-169 of SHBs, especially those within the “a” determinant between aa 124-147, can lead to conformational changes of HBsAg. Mutated HBsAg is poorly detected by immunoassays based on monoclonal antibodies, contributing to some cases of OBI. OBI: Occult hepatitis B virus infection; HBsAg: Hepatitis B surface antigen.
- Citation: Wu CC, Chen YS, Cao L, Chen XW, Lu MJ. Hepatitis B virus infection: Defective surface antigen expression and pathogenesis. World J Gastroenterol 2018; 24(31): 3488-3499
- URL: https://www.wjgnet.com/1007-9327/full/v24/i31/3488.htm
- DOI: https://dx.doi.org/10.3748/wjg.v24.i31.3488