Copyright
©The Author(s) 2017.
World J Gastroenterol. Aug 7, 2017; 23(29): 5313-5323
Published online Aug 7, 2017. doi: 10.3748/wjg.v23.i29.5313
Published online Aug 7, 2017. doi: 10.3748/wjg.v23.i29.5313
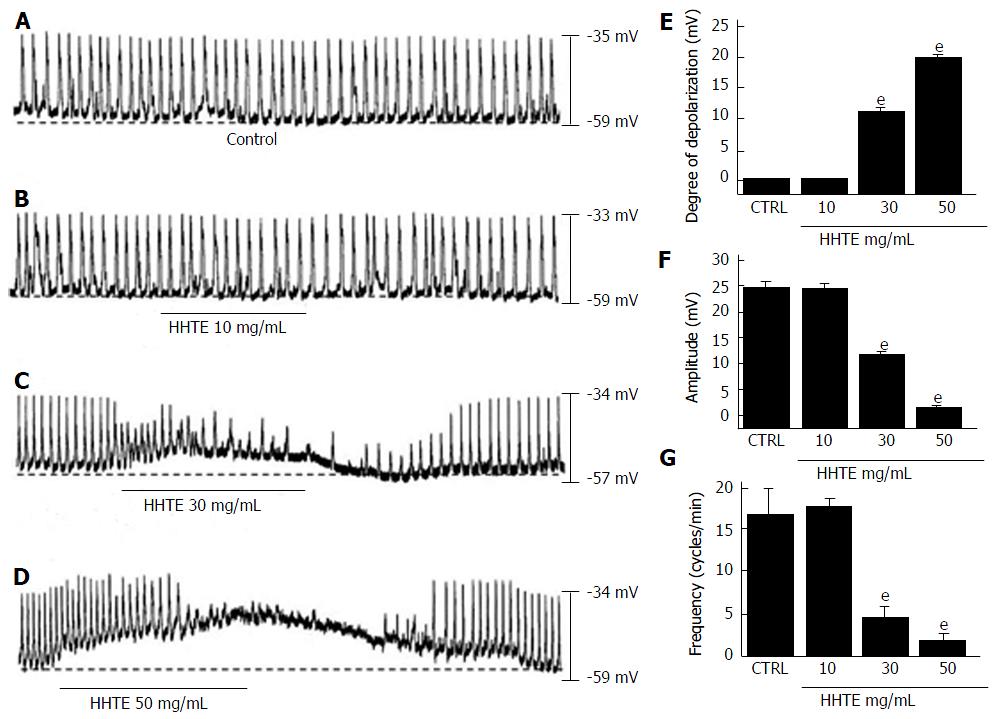
Figure 1 Effects of water extract of Hwangryunhaedok-tang on the pacemaker potentials of cultured interstitial cells of Cajal from murine small intestine.
A-D: HHTE (0-50 mg/mL) induced pacemaker potential depolarizations and decreased amplitudes in a concentration-dependent manner. Responses to HHTE are summarized (E-G). Bars indicate the means ± SEMs. eP < 0.001: significantly different from the control. CTRL: Control; HHTE: Water extract of Hwangryunhaedok-tang.
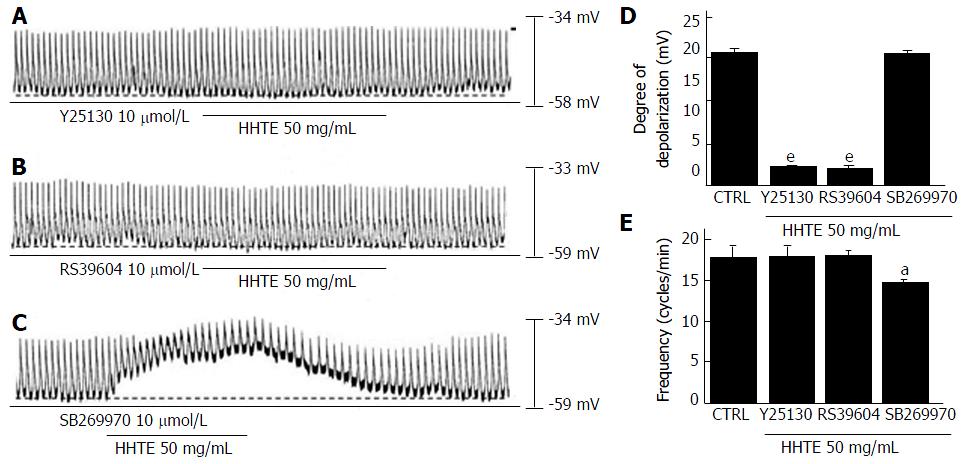
Figure 2 Effects of 5-HT receptor antagonists on water extract of Hwangryunhaedok-tang-induced pacemaker potential depolarizations in the cultured interstitial cells of Cajal of murine small intestine.
A: Y25130 (a 5-HT3 receptor antagonist) inhibited HHTE-induced responses. B: A 5-HT4 receptor antagonist, RS39604, blocked HHTE-induced responses. C: A 5-HT7 receptor antagonist, SB269970, did not block HHTE-induced responses. D and E responses to 5-HT receptor antagonists are summarized. Bars indicate the means ± SEMs. aP < 0.05 and eP < 0.001: Significantly different from nontreated controls. CTRL: Control; HHTE: Water extract of Hwangryunhaedok-tang.
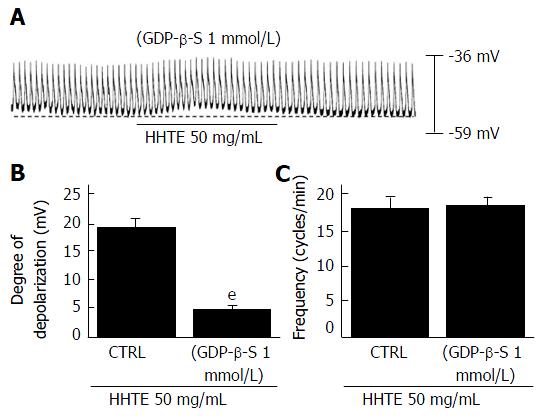
Figure 3 Effects of GDP-β-S on water extract of Hwangryunhaedok-tang-induced pacemaker potential depolarizations in cultured interstitial cells of Cajal.
A: When GDP-β-S (1 mmol/L) was applied in the pipette, HHTE did not depolarize the pacemaker potential. B and C: HHTE-induced responses are summarized. Bars indicate the means ± SEMs. eP < 0.001: Significantly different from nontreated controls. CTRL: Control; HHTE: Water extract of Hwangryunhaedok-tang.
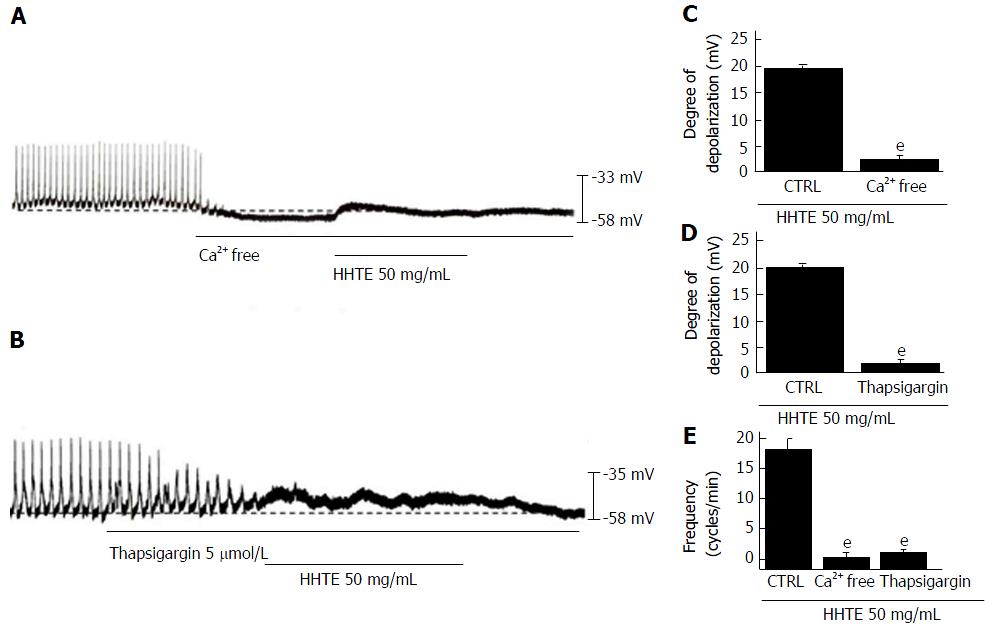
Figure 4 Effects of external and internal Ca2+ on water extract of Hwangryunhaedok-tang-induced pacemaker potential depolarization in cultured interstitial cells of Cajal from murine small intestine.
A: In external Ca2+-free conditions, the pacemaker potentials were abolished and HHTE had no effect. B: Thapsigargin abolished the pacemaker potentials and HHTE had no effects; C-E: HHTE-induced pacemaker potential depolarizations are summarized. Bars represent the means ± SEMs. eP < 0.001: Significantly different from untreated controls. CTRL: Control; HHTE: Water extract of Hwangryunhaedok-tang.
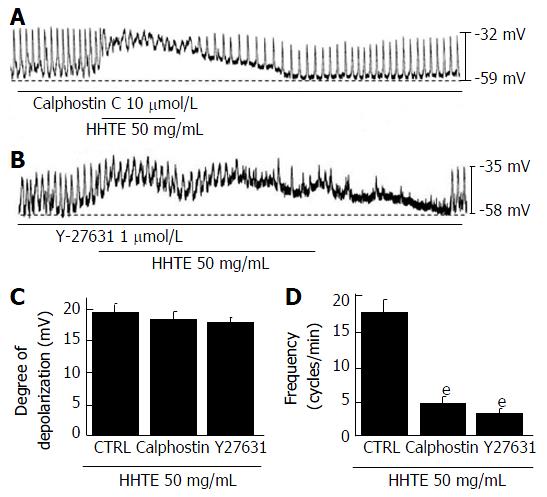
Figure 5 Effects of protein kinase C inhibitor and Rho kinase inhibitor on water extract of Hwangryunhaedok-tang-induced pacemaker potential depolarizations in cultured interstitial cells of Cajal from murine small intestine.
Both (A) calphostin C (a PKC inhibitor) and (B) Y27631 (a Rho kinase inhibitor) had no effects on the pacemaker potentials; under these conditions, HHTE induced pacemaker potential depolarizations. C and D responses to HHTE in the presence of calphostin C or Y27631 are summarized. Bars represent the means ± SEMs. eP < 0.001: significantly different from the nontreated control. CTRL: Control; HHTE: Water extract of Hwangryunhaedok-tang; PKC: Protein kinase C.
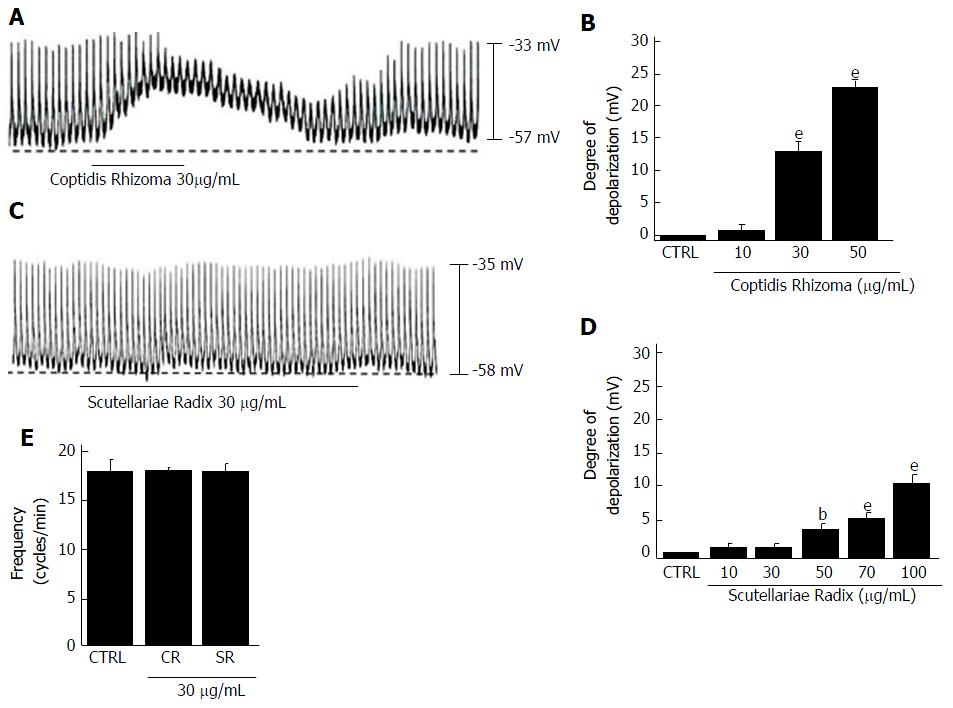
Figure 6 Effects of Coptidis Rhizoma and Scutellariae Radix on pacemaker potentials in cultured interstitial cells of Cajal from murine small intestine.
A: Pacemaking activities of ICCs exposed to Coptidis Rhizoma (30 μg/mL) in current-clamp mode (I = 0); B: Coptidis Rhizoma (10-50 μg/mL) concentration-dependently induced pacemaker potential depolarization. Responses to Coptidis Rhizoma are summarized; C: Pacemaking activities of ICCs exposed to Scutellariae Radix (30 μg/mL) in the current-clamp mode (I = 0); D: Scutellariae Radix (10-100 μg/mL) concentration-dependently induced pacemaker potential depolarization. Responses to Scutellariae Radix are summarized; E: Frequency changes to Coptidis Rhizoma and Scutellariae Radix are summarized. Bars represent the means ± SEMs. bP < 0.01 and eP < 0.001: Significantly different from the control. CTRL: Control; CR: Coptidis Rhizoma; SR: Scutellariae Radix; ICCs: Interstitial cells of Cajal.
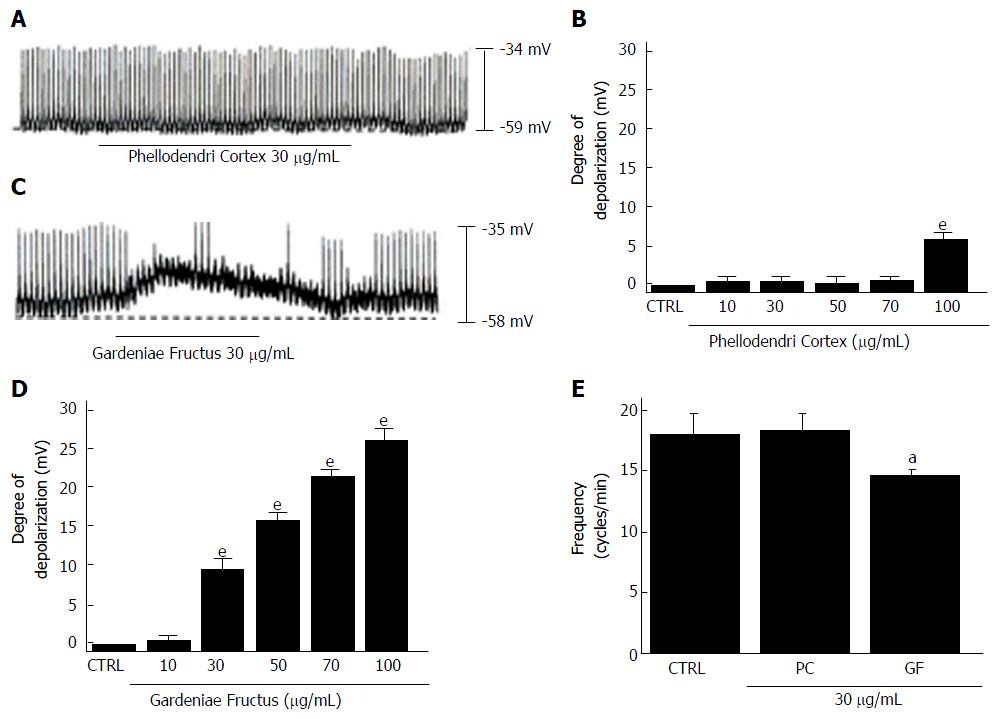
Figure 7 Effects of Phellodendri Cortex and Gardeniae Fructus on pacemaker potentials in cultured interstitial cells of Cajal from murine small intestine.
A: Pacemaking activities of ICCs exposed to Phellodendri Cortex (30 μg/mL) in the current-clamp mode (I = 0); B: Phellodendri Cortex (10-100 μg/mL) concentration-dependently induced pacemaker potential depolarization. Responses to Phellodendri Cortex are summarized; C: Pacemaking activities of ICCs exposed to Gardeniae Fructus (30 μg/mL) in the current-clamp mode (I = 0); D: Gardeniae Fructus (10-100 μg/mL) concentration-dependently induced pacemaker potential depolarizations. Responses to Gardeniae Fructus are summarized; E: Frequency changes to Phellodendri Cortex and Gardeniae Fructus are summarized. Bars represent the means ± SEMs. aP < 0.05 and eP < 0.001: Significantly different from the control. CTRL: Control; PC: Phellodendri Cortex. GF: Gardeniae Fructus.
- Citation: Kim HJ, Lee GS, Kim H, Kim BJ. Hwangryunhaedok-tang induces the depolarization of pacemaker potentials through 5-HT3 and 5-HT4 receptors in cultured murine small intestine interstitial cells of Cajal. World J Gastroenterol 2017; 23(29): 5313-5323
- URL: https://www.wjgnet.com/1007-9327/full/v23/i29/5313.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i29.5313