Copyright
©The Author(s) 2017.
World J Gastroenterol. Jul 28, 2017; 23(28): 5068-5085
Published online Jul 28, 2017. doi: 10.3748/wjg.v23.i28.5068
Published online Jul 28, 2017. doi: 10.3748/wjg.v23.i28.5068
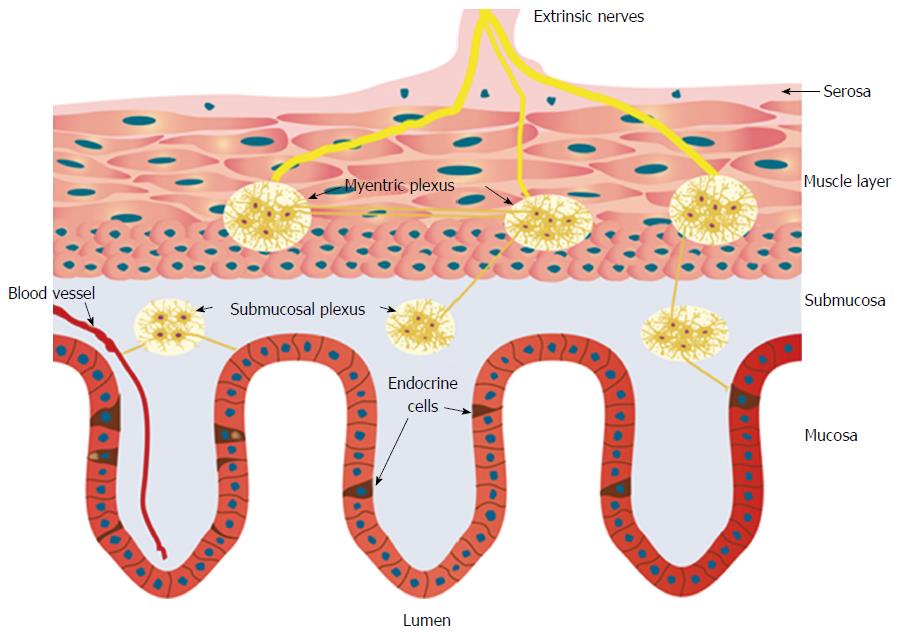
Figure 1 Schematic of the gastrointestinal neuroendocrine regulatory system.
The neuroendocrine regulatory system (NES) comprises gastrointestinal (GI) endocrine cells in the mucosa and the enteric nervous system (ENS). The ENS consists of two plexi: one located in the submucosa (the submucosa plexus) and one situated between the longitudinal and circular muscle layers (the myenteric plexus). The GI endocrine cells integrate and interact with each other and with the ENS. The GI NES is an independent system that regulates most of the GI functions and is modulated by the central nervous system.
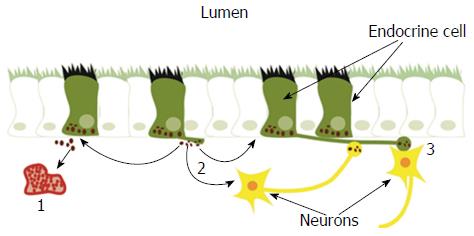
Figure 2 Gastrointestinal endocrine cells have sensory microvilli projecting into the gastrointestinal lumen that register and respond to luminal stimuli by releasing their hormones into the lamina propria.
The released hormones exert their effects via three modes of action: (1) entering the circulating blood and reaching distant targets (endocrine mode); (2) acting locally on nearby structures (paracrine mode); or (3) via synaptic activity. Reproduced from reference 46 with permission from the authors and the publisher.
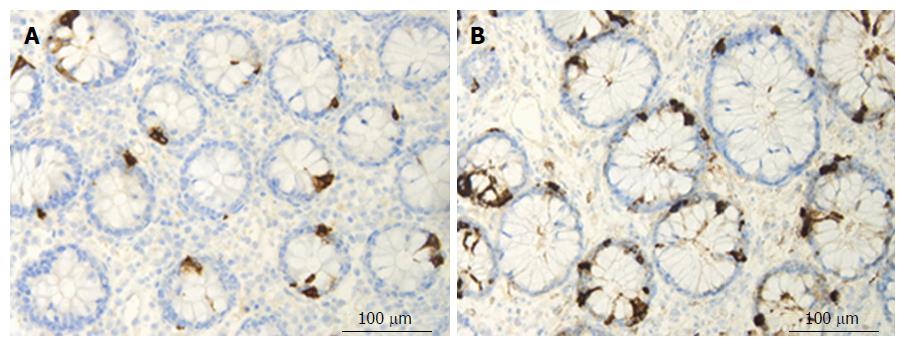
Figure 3 Colonic chromogranin A immunoreactive cells in a control subject (A) and in a patient with lymphocytic colitis (B).
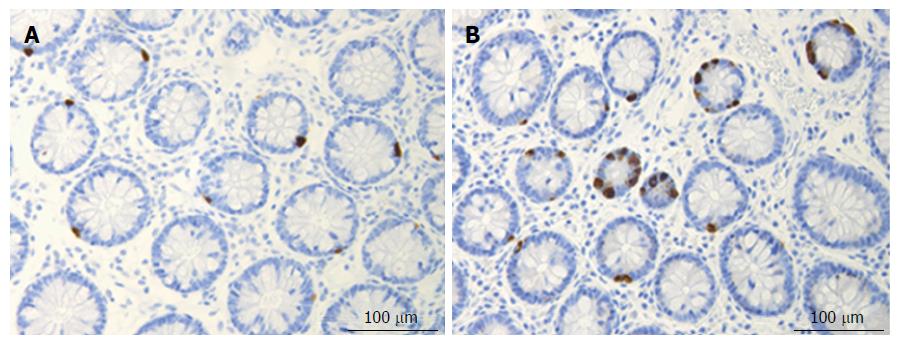
Figure 4 Colonic serotonin cells in a control subject (A) and in a patient with lymphocytic colitis (B).
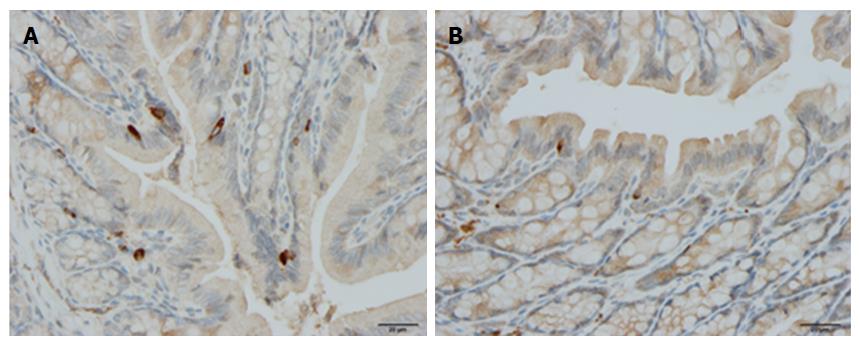
Figure 5 Colonic somatostatin immunoreactive cells in a control rat (A) and in a rat with dextran sulfate sodium-induced colitis (B).
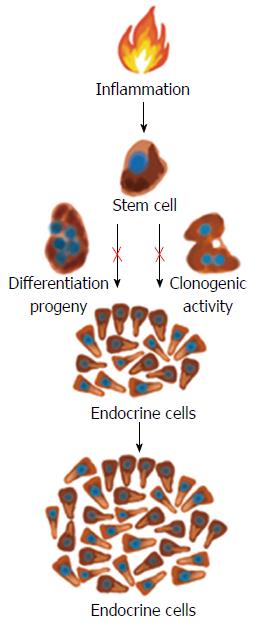
Figure 6 Proinflammatory substances such as cytokines may act on the intestinal stem cells and increase their clonogenic and differentiation progeny so that the density of intestinal endocrine cells increases during active inflammation.
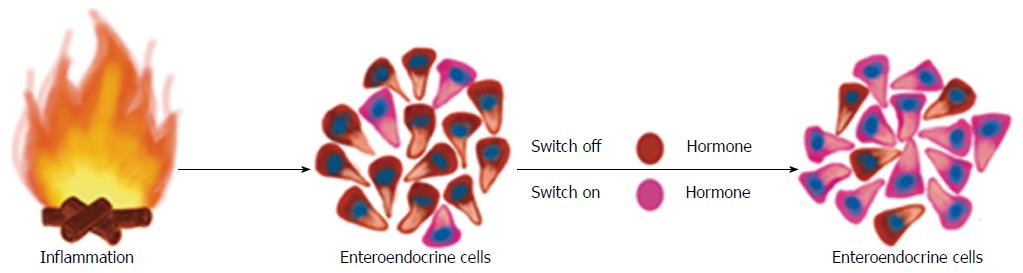
Figure 7 During the inflammatory process, several proinflammatory substances may act on the mature endocrine cells so that they switch off the expression of a certain neuroendocrine peptides/amines and switch on the expression of another neuroendocrine peptides/amines.
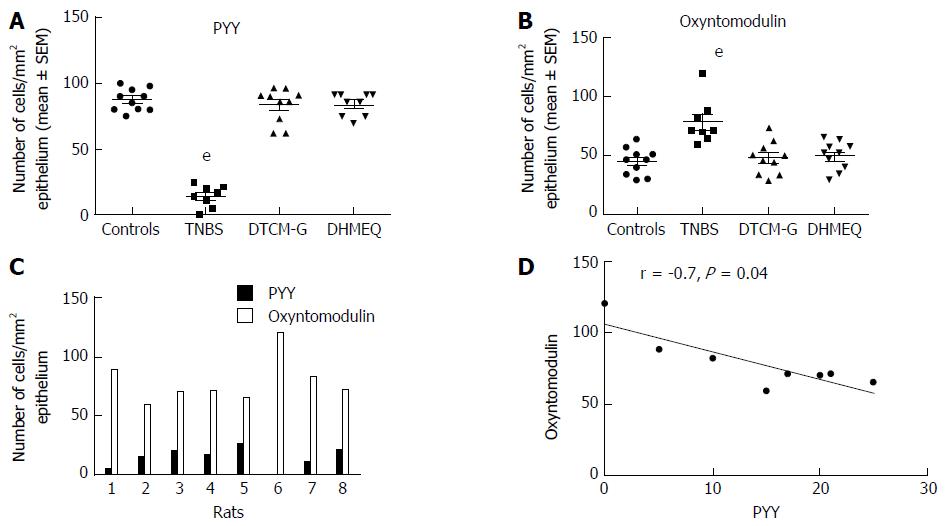
Figure 8 Colonic densities of (A) peptide YY-positive cells and (B) oxyntomodulin (enteroglucagon)-positive cells in control rats, in rats with trinitrobenzene sulfonic acid (TNBS)-induced colitis, and in rats with TNBS-induced colitis treated with 3-[(dodecylthiocarbonyl)-methyl]-glutarimide (DTCM-G, an activator protein-1 inhibitor) and dehydroxymethylepoxyquinomicin (DHMEQ, a nuclear factor-κB inhibitor).
Densities of PYY-positive and oxyntomodulin-positive cells in each rat of the TNBS group (C), and their correlation (D). eP < 0.001 vs controls. Reproduced from reference 274 with permission from the authors and the publisher. PYY: Peptide YY.
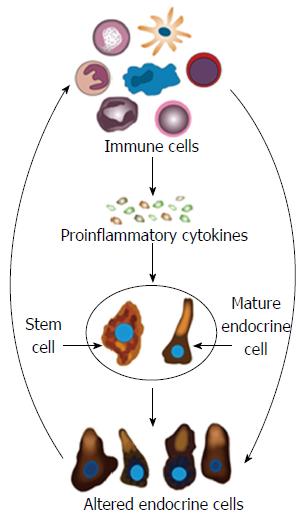
Figure 9 Schematic of the possible mechanisms underlying the changes in gastrointestinal endocrine cells in inflammatory bowel disease.
In active inflammatory bowel disease, the immune cells (IC) produce proinflammatory cytokines and other substances that affect the gastrointestinal (GI) stem cells and mature endocrine cells. Thus, abnormal clonogenic and differentiation activities are induced in stem cells. Moreover, the mature endocrine cells switch off the expression of certain hormones in favor of switching on the synthesis of other hormones. This would change the GI endocrine cell density and alter the proportions of the various endocrine cell types. The NPA released by the altered endocrine cells would in return affect the IC via their NPA receptors.
- Citation: El-Salhy M, Solomon T, Hausken T, Gilja OH, Hatlebakk JG. Gastrointestinal neuroendocrine peptides/amines in inflammatory bowel disease. World J Gastroenterol 2017; 23(28): 5068-5085
- URL: https://www.wjgnet.com/1007-9327/full/v23/i28/5068.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i28.5068