Copyright
©The Author(s) 2017.
World J Gastroenterol. Jun 28, 2017; 23(24): 4390-4398
Published online Jun 28, 2017. doi: 10.3748/wjg.v23.i24.4390
Published online Jun 28, 2017. doi: 10.3748/wjg.v23.i24.4390
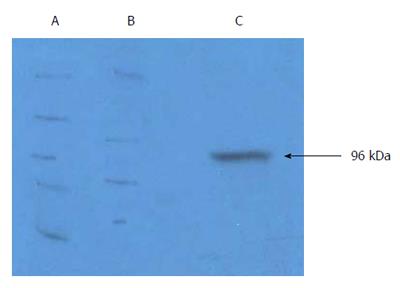
Figure 1 Characterization of purified gp96-GC antigen peptide complexes.
A: Western blot of gp96-GC antigen peptide complexes from KATOIII cells; B: KATOIII GC cell proteins; C: Purified eluted fraction from KATOIII cells using diethyl-aminoethyl-Sepharose anion exchange chromatography.
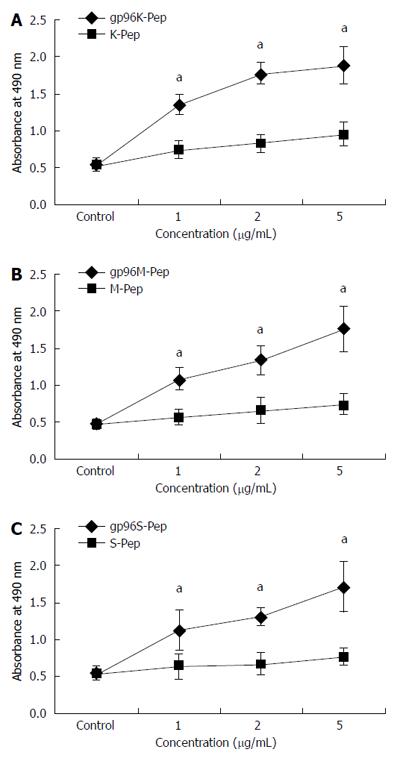
Figure 2 Proliferation assays of gastric cancer cell strains treated with purified gp96-peptide complexes.
MTS assays were performed for: A: KATOIII source GP96-gastric cancer (GC) antigen peptide complexes (gp96K-Pep) vs KATOIII source antigen peptide (K-Pep); B: MKN-28 source GP96-GC antigen peptide complexes (gp96M-Pep) vs MKN-28 source antigen peptide (M-Pep); C: SGC-7901 source gp96-GC antigen peptide complexes (p96S-Pep) vs SGC-7901 source antigen peptide (S-Pep). aP < 0.05.
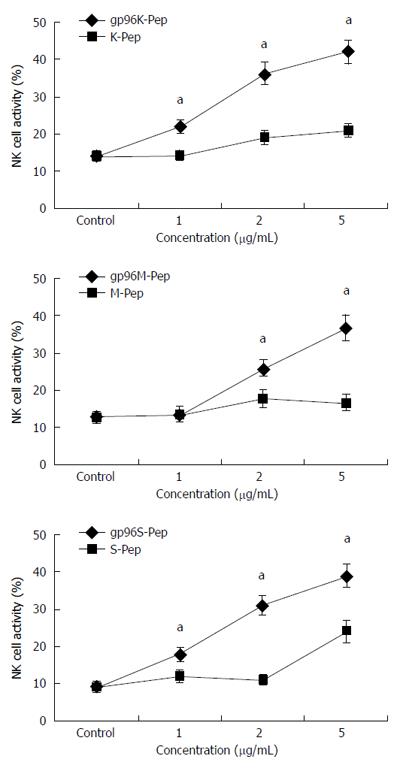
Figure 3 Activity of natural killer cells assayed in the presence of gp96-peptide complexes.
At an effector to target cell ratio of 10:1, natural killer cells were incubated with three different gastric cancer (GC) cell strains in the presence of 1, 2 and 5 μg/mL gp96-peptide complexes or gp96 purified from Ad-gp96 expression. NK cell activity for (A) KATOIII, (B) MKN-28 and (C) SGC-7901 cells incubated with gp96-peptide complexes (gp96K-Pep, gp96M-Pep and gp96S-Pep) vs GC cell source antigen peptide (K-Pep, M-Pep and S-Pep) for each of the three cell lines, respectively. aP < 0.05.
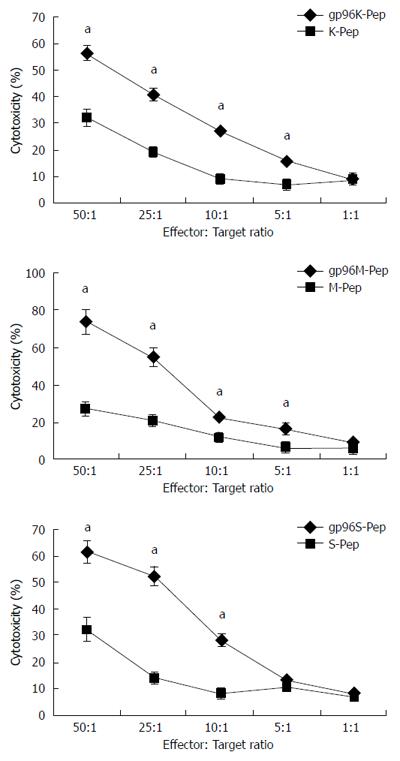
Figure 4 Cytotoxicity assays for T cells.
Cytotoxic T lymphocytes (CTLs) were incubated with KATOIII, MKN-28 or SGC-7901 cells in the presence of gp96-peptide complexes (gp96K-Pep, gp96M-Pep and gp96S-Pep) vs gastric cancer cell source antigen peptide (K-Pep, M-Pep and S-Pep) at the target to effector cell ratios indicated. aP < 0.05.
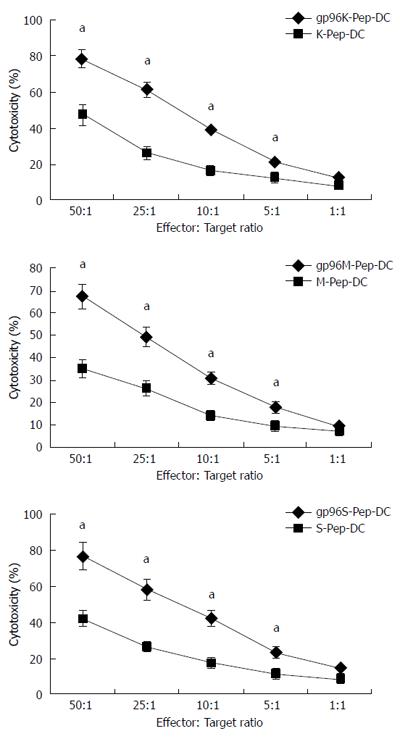
Figure 5 Cytotoxicity assays for dendritic cells.
Dendritic cells (DCs) were incubated with KATOIII, MKN-28 and SGC-7901 cells in the presence of gp96-peptide complexes (gp96K-Pep-DC, gp96M-Pep-DC and gp96S-Pep-DC) vs gastric cancer cell source antigen peptide (K-Pep-DC, M-Pep-DC and S-Pep-DC) at the target to effector cell ratios indicated. aP < 0.05.
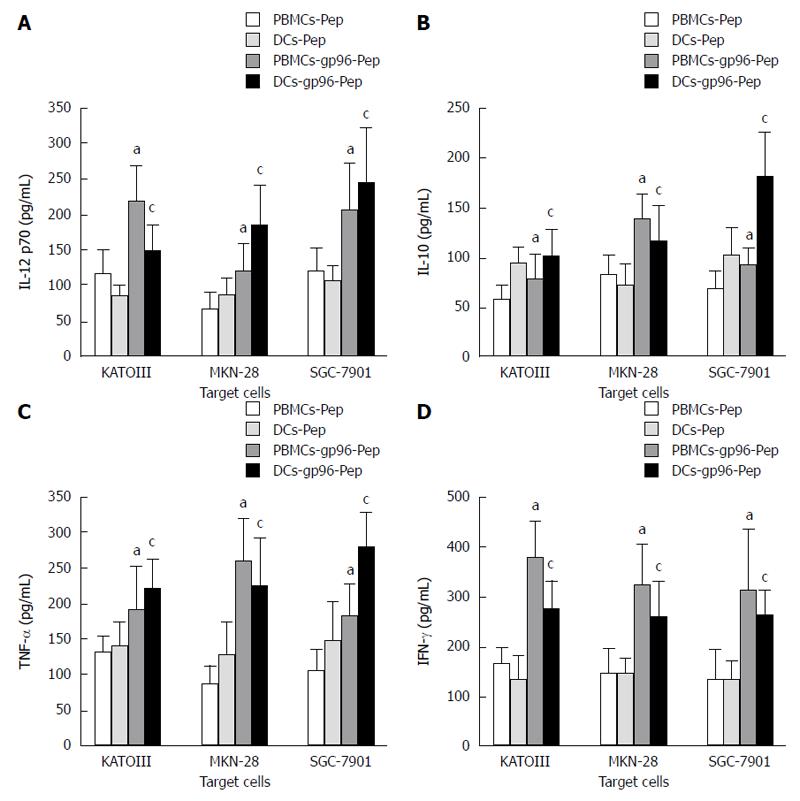
Figure 6 Cytokine-specific sandwich enzyme-linked immunosorbent assays.
Cytokine expression by peripheral blood mononuclear cells (PBMCs) and dendritic cells (DCs) in the presence or absence of gp96-peptide complexes purified from KATOIII, MKN-28 and SGC-7901 cells. A: IL-12 p70; B: IL-10; C: TNF-α; D: IFN-γ. PBMCs cultured with gp96-GC peptide complexes vs PBMCs cultured with gastric cancer (GC) peptide, aP < 0.05; DCs cultured with gp96-GC peptide complexes vs DCs cultured with GC peptide, cP < 0.05.
- Citation: Lu WW, Zhang H, Li YM, Ji F. Gastric cancer-derived heat shock protein-gp96 peptide complex enhances dendritic cell activation. World J Gastroenterol 2017; 23(24): 4390-4398
- URL: https://www.wjgnet.com/1007-9327/full/v23/i24/4390.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i24.4390