Copyright
©The Author(s) 2017.
World J Gastroenterol. Jan 14, 2017; 23(2): 297-305
Published online Jan 14, 2017. doi: 10.3748/wjg.v23.i2.297
Published online Jan 14, 2017. doi: 10.3748/wjg.v23.i2.297
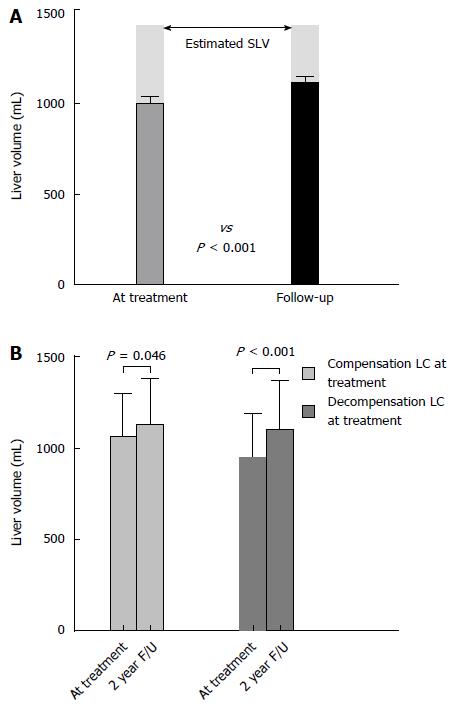
Figure 1 Change of liver volume during long-term oral nucleos(t)ide analogues therapy.
A: The overall change of liver volume from treatment initiation to second-year follow-up. Liver volume was 993.8 mL ± 242.8 mL at treatment initiation and 1108.1 mL ± 263.3 mL at follow-up. There was 114.3 mL ± 167.8 mL (12.9% ± 17.9%) of volume increase during the two years of treatment period. Compared to the estimated standard liver volume (SLV), as described by Yu et al[14]. which was 1436.0 mL ± 170.2 mL (shaded in gray), measured baseline liver volume in this study was 70.8% and follow-up volume was improved to 78.0% of estimated SLV; B: Comparison of changes of liver volume according to the presence of decompensation at treatment initiation. LC: Liver cirrhosis; F/U: Follow-up.
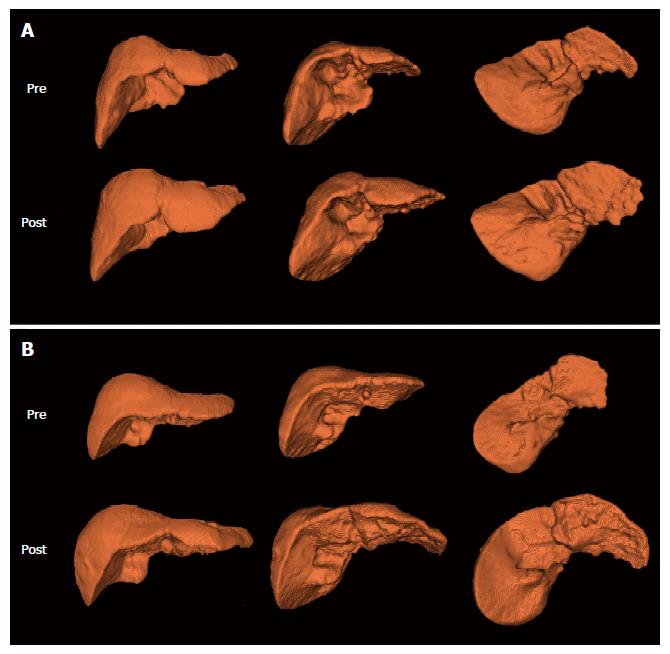
Figure 2 Representative 3-dimentional reconstruction images in patients with (A) compensated liver cirrhosis and (B) decompensated liver cirrhosis.
Among 6 images for each patient, upper images are baseline (pre-NUCs therapy) images and lower images are follow-up (post-NUCs therapy) images in different views (anterior, anteroinferomedial, and posteroinferomedial). NUCs: Nucleos(t)ide analogues.
- Citation: Lee CH, Kim IH, Moon JC, Seo SY, Kim SH, Kim SW, Lee SO, Lee ST, Kim DG, Yang JD, Yu HC. 3-Dimensional liver volume assessment in patients with hepatitis B virus-related liver cirrhosis during long-term oral nucleos(t)ide analogues therapy. World J Gastroenterol 2017; 23(2): 297-305
- URL: https://www.wjgnet.com/1007-9327/full/v23/i2/297.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i2.297