Copyright
©The Author(s) 2017.
World J Gastroenterol. Apr 7, 2017; 23(13): 2396-2403
Published online Apr 7, 2017. doi: 10.3748/wjg.v23.i13.2396
Published online Apr 7, 2017. doi: 10.3748/wjg.v23.i13.2396
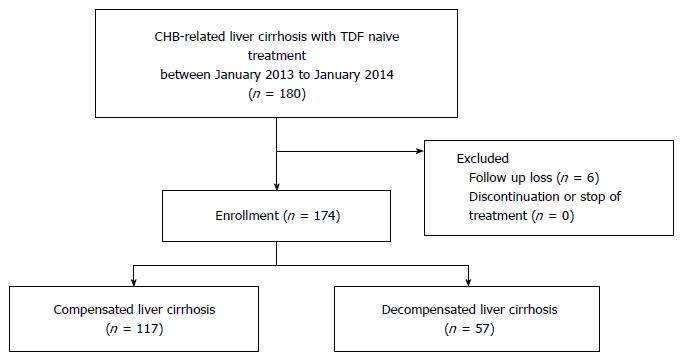
Figure 1 Flow chart of the enrolled participants.
CHB: Chronic hepatitis B; TDF: Tenofovir disoproxil fumarate.
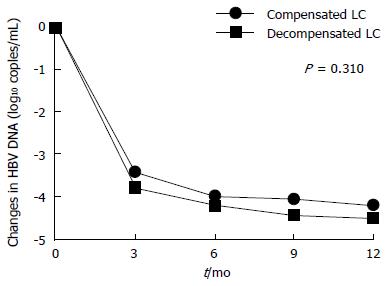
Figure 2 The mean changes in serum HBV DNA levels of the decompensated and compensated cirrhosis group.
There were no significant differences in the reduction of serum HBV DNA levels between two groups (P = 0.310). LC: Liver cirrhosis.
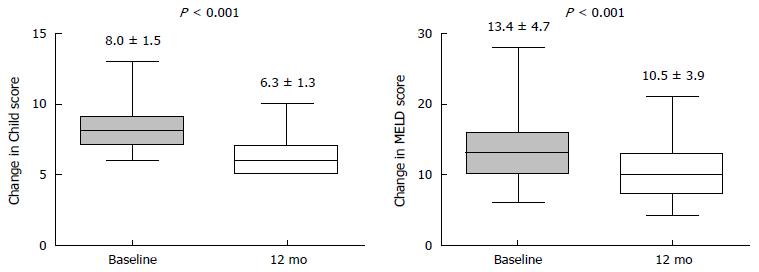
Figure 3 The changes of the Child-Turcotte-Pugh and model for end-stage liver disease scores in the decompensated group after tenofovir disoproxil fumarate treatment for 12 mo.
The mean Child-Turcotte-Pugh (CTP) score (8.0 ± 1.5 vs 6.3 ± 1.3) and model for end-stage liver disease (MELD) scores (13.4 ± 4.7 vs 10.5 ± 3.9) improved after 12 mo of tenofovir disoproxil fumarate treatment than at baseline (P < 0.001 for all). CTP: Child-Turcotte-Pugh; MELD: Model for End-stage Liver Disease.
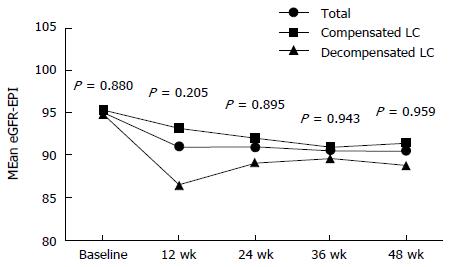
Figure 4 The changes of creatinine clearance (eGFR) during the tenofovir disoproxil fumarate therapy.
Of seven patients with increased serum creatinine more than 0.5 mg/dL, three were in compensated group and four were in decompensated group (2.5% vs 7.0%, P < 1.000). There were no statistically significant differences in the changes of creatinine clearance between the decompensated and the compensated group during treatment. LC: Liver cirrhosis.
- Citation: Lee SK, Song MJ, Kim SH, Lee BS, Lee TH, Kang YW, Kim SB, Song IH, Chae HB, Ko SY, Lee JD. Safety and efficacy of tenofovir in chronic hepatitis B-related decompensated cirrhosis. World J Gastroenterol 2017; 23(13): 2396-2403
- URL: https://www.wjgnet.com/1007-9327/full/v23/i13/2396.htm
- DOI: https://dx.doi.org/10.3748/wjg.v23.i13.2396