Copyright
©The Author(s) 2016.
World J Gastroenterol. Dec 21, 2016; 22(47): 10275-10286
Published online Dec 21, 2016. doi: 10.3748/wjg.v22.i47.10275
Published online Dec 21, 2016. doi: 10.3748/wjg.v22.i47.10275
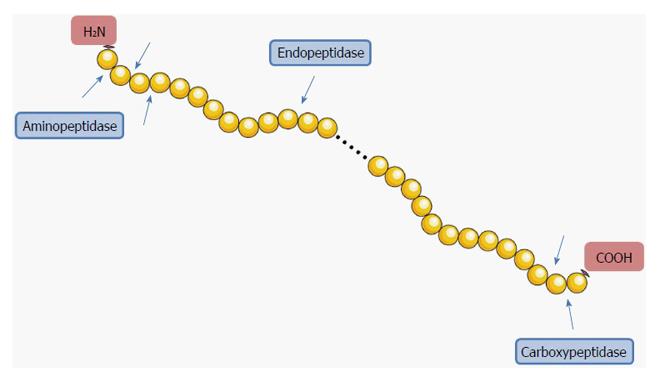
Figure 1 Simplified representation of the concept of endo- and exopeptidases.
Endopeptidases cleave internal peptide bonds. Exopeptidases cleave terminal peptide bonds; they can be subdivided into amino- and carboxypeptidases according to the position of the cleavage of the peptide bond. Aminopeptidases cleave at amino (NH2) terminal bonds, while carboxypeptidases cleave at carboxy (COOH) terminal bonds. Image constructed using the Servier Image Bank.
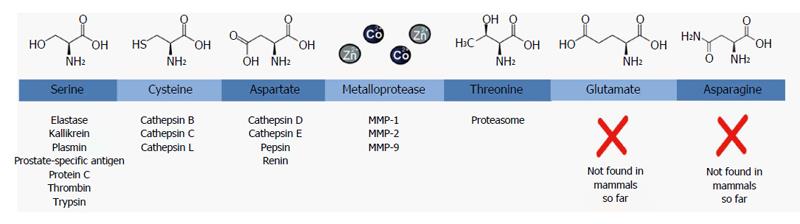
Figure 2 Classification of proteases based on the chemical structure of their active site.
For each class, the chemical structure of the core residue in their active site is shown on top and a few examples with medical relevance of proteases belonging to that family are displayed below. MMP: Matrix metalloprotease.
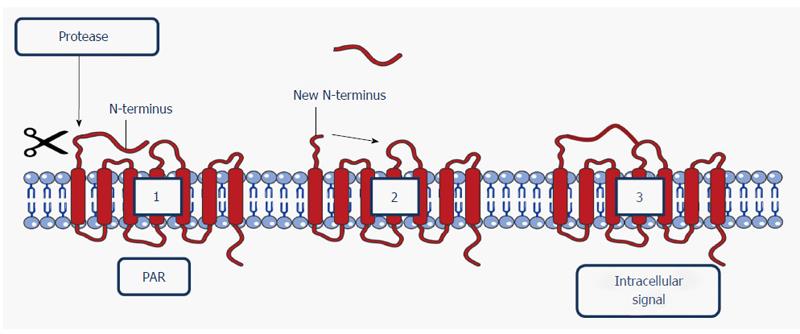
Figure 3 Schematic representation of the activation of a protease-activated receptor.
A protease cleaves the N-terminal domain (1), releasing a new N-terminus (2). The new N-terminus binds to the receptor as a tethered ligand, providing an intracellular signal (3). Image constructed using the Servier Image Bank. N-terminus: Amino-terminus; PAR: Protease-activated receptor.
- Citation: Ceuleers H, Van Spaendonk H, Hanning N, Heirbaut J, Lambeir AM, Joossens J, Augustyns K, De Man JG, De Meester I, De Winter BY. Visceral hypersensitivity in inflammatory bowel diseases and irritable bowel syndrome: The role of proteases. World J Gastroenterol 2016; 22(47): 10275-10286
- URL: https://www.wjgnet.com/1007-9327/full/v22/i47/10275.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i47.10275