Copyright
©The Author(s) 2016.
World J Gastroenterol. Oct 28, 2016; 22(40): 9035-9038
Published online Oct 28, 2016. doi: 10.3748/wjg.v22.i40.9035
Published online Oct 28, 2016. doi: 10.3748/wjg.v22.i40.9035
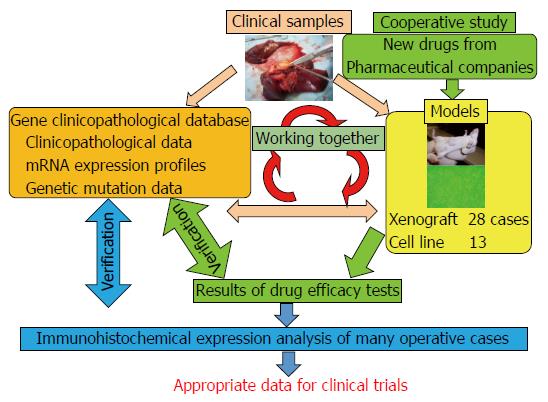
Figure 1 Relationship between our materials and databases.
There are three key factors: clinical samples, databases, and biliary tract carcinoma (BTC) models. Both the models and the databases are derived from the clinical samples. These databases comprise “clinicopathological data”, “mRNA expression profiles”, and “genetic mutation data”. BTC models are “xenograft models” and “cell lines”. These models are used for cooperative studies with pharmaceutical companies for translational research. For example, they provide us with new anti-cancer drugs, and we can perform drug efficacy tests. If necessary, we can also perform an immunohistochemical expression analysis. Then, we can compare the results of the analysis with those in the databases and validate them. After these steps, we can provide appropriate data to clinicians. Together, these databases and materials make translational research far more detailed and suitable for clinical trials.
- Citation: Ojima H, Yamagishi S, Shimada K, Shibata T. Establishment of various biliary tract carcinoma cell lines and xenograft models for appropriate preclinical studies. World J Gastroenterol 2016; 22(40): 9035-9038
- URL: https://www.wjgnet.com/1007-9327/full/v22/i40/9035.htm
- DOI: https://dx.doi.org/10.3748/wjg.v22.i40.9035